Abstract
Currently, several non-invasive modalities, including MRI and PET, are being investigated to identify early intestinal inflammation, longitudinally monitor disease status, or detect dysplastic changes in patients with inflammatory bowel disease (IBD). Here, we assess the applicability and utility of multispectral optoacoustic tomography (MSOT) in evaluating the presence and severity of colitis. Mice with bacterial colitis demonstrated a temporally associated increase in mesenteric and colonic vascularity with an increase in mean signal intensity of oxygenated hemoglobin (p=0.004) by MSOT two days after inoculation. These findings were significantly more prominent 7 days after inoculation, with increased mean signal intensity of oxygenated hemoglobin (p=0.0002) and the development of punctate vascular lesions on the colonic surface, which corresponded to changes observed on colonoscopy as well as histology. With improvements in depth of tissue penetration, MSOT may hold potential as a sensitive, accurate, non-invasive imaging tool in evaluation of patients with IBD.
Introduction
Inflammatory bowel disease (IBD) has grown increasingly prevalent over the last half century, with an annual incidence of approximately 250–300 per 100,000 persons in North America (1). Diagnosis usually involves a combination of factors identified in a patient’s history and on physical examination, stool studies, and, ultimately, colonoscopy with multiple biopsies (2). Furthermore, given the increased risk of colorectal cancer in patients with IBD, current practice guidelines recommend annual screening colonoscopies 8–10 years after onset of disease to assess for dysplastic changes and status of disease (3). In addition to being especially uncomfortable for patients with inflammatory bowel disease, colonoscopy represents an invasive procedure that carries risk from both sedation or anesthesia and colonic perforation. To date, no non-invasive imaging modalities have been developed that accurately identify early inflammatory changes in patients’ small bowel and colon, that can be used longitudinally to monitor the status of disease, or detect changes concerning for malignancy.
Multispectral optoacoustic tomography (MSOT) represents a novel modality that detects sound waves resulting from specific molecular excitation by light. Specifically, laser stimulation of tissues results in generation and emission of ultrasound waves. Ultrasound waves demonstrate significantly decreased scatter compared to light waves prior to reaching the detecting transducer (4). Thus, MSOT retains speed and sensitivity while having an improved signal to noise ratio compared with conventional radiographic imaging modalities (e.g. computed tomography (CT) and magnetic resonance imaging (MRI) (4–11). To date, MSOT has demonstrated resolution at 78.9 μm (5,6). Furthermore, it can detect changes in tissue architecture and the presence of oxygenated and deoxygenated hemoglobin (5–10) permitting evaluation of changes in structure and vascularity, common in IBD. Recently, MSOT instrumentation has evolved and includes the potential for combination of detecting both optoacoustic and ultrasound signals which increases its radiological capabilities. Currently, MSOT has been used to effectively image tumor xenografts as well as several orthotopic tumor models, including pancreatic adenocarcinoma (6–8), but not to specifically assess inflammatory or dysplastic changes in the bowel in murine models.
Colonization with enterotoxic Bacteroides fragilis (ETBF), a pathogenic variant of a human intestinal commensal organism, has been implicated in the pathogenesis of inflammatory bowel disease (12–13.) Indeed, wild type C57B/6 mice inoculated with ETBF after antibiotic-mediated depletion of intestinal flora can either initiate colitis or worsen susceptibility to colitis induced by other means (12–13). Like colitis observed in IBD patients, the inflammation persists over time and comprises both local and systemic components. Here, we utilize this model of ETBF-induced colitis in C57B/6 mice to evaluate the ability of MSOT to detect intestinal and colonic inflammation.
Methods
Mice
C57B/6 mice (Jackson Labs, USA) were bred in accordance with University of Louisville (UofL) Institutional Animal Care and Use Committee (IACUC) guidelines. 6–7 week old mice of both genders were used for all experiments. A total of 9 mice were used for experiments. All experiments were conducted in accordance with UofL IACUC guidelines. For an experimental schematic, please see Supplemental Figure 1.
Bacteria. Enterotoxic Bacteroides fragilis strain 86-5443-2-2 was cultured under anaerobic conditions on BHI + clindamycin agar plates. Plates were streaked with bacterial stock stored in glycerol and subsequently incubated in under anaerobic conditions at 37°C. After 48 hours, bacteria were harvested and suspended in liquid BHI + clindamycin broth before being incubated under anaerobic conditions for another 24 hours.
Induction of Colitis
To deplete enteric pathogen load and facilitate ETBF colonization, mice were administered clindamycin (0.1 g/L) and streptomycin (5 g/L) dissolved in drinking water for 4 days prior to bacterial inoculation. Approximately 1 × 108 bacteria suspended in 200 μL phosphate buffered saline were then administered via oral gavage into the gastrointestinal tract of these mice. Mice that received neither antibiotics nor bacteria were used as controls. All mice were fed casein-based, low-anthacyanin chow ad libitum (TekLad 2920X, Envigo, Huntington, UK) and monitored closely for signs of dehydration and provided daily with electrolyte-rich gel supplements.
Imaging and statistical comparison
Multispectral optoacoustic tomographic (MSOT) imaging was performed as previously described (6). Briefly, mice were anesthetized with 1.6% isoflurane inhalant delivered in 0.8 L medical air and 0.1 L O2, then depilated using a combination of shaving and application of Nair with aloe (Church & Dwight Co, Princeton, NJ, USA), which was removed with moist gauze. Mice were subsequently placed in the prone position and imaged from superior thorax to inferior pelvis using the MSOT system InVision 256TF (iThera Medical, Munich, Germany) using wavelengths of 680, 710, 730, 740, 760, 770, 780, 800, 850, and 900 nm with 25 averages per wavelength and an acquisition time of 10 μs per frame. Accurate subject positioning within the MSOT device was ensured using preview Mice were evaluated prior to bacterial inoculation (untreated normal control), 2 days after bacterial inoculation, and 7 days after bacteria inoculation. Images were reconstructed via backprojection with 75 μm resolution. MSOT values for oxygenated and deoxygenated hemoglobin were determined using MSOT imaging software (ViewMSOT 3.5) and compared using linear regression (JMP software (JMP, SAS Institute Inc., Cary, NC)). Region of interest analysis was utilized to determine oxy-and deoxy-hemoglobin separately (6) within the colon using a 3.5 mm2 ellipse on 4 regions of the mouse colon per time point. Mean oxygenated hemoglobin signal intensity among the 4 regions was determined and was compared using a one-way ANOVA test with Tukey’s HSD using JMP software (JMP, SAS Institute Inc., Cary, NC). Differences were considered significant for p<0.05.
Colonoscopy and statistical comparison
Mice were injected intraperitoneally with 0.1 mL/20 g mouse weight of a ketamine/xylazine cocktail (87.5 mg/kg ketamine + 12.5 mg/kg xylazine). After achieving an adequate level of anesthesia, an 8 French pediatric cystoscope was introduced into the mouse anus and advanced proximally until the scope could no longer be advanced (12). The colonoscope was then withdrawn slowly. The Murine Endoscopic Index of Colitis Severity (MEICS) was used to quantify colitis severity (Table 1). Colitis severity score was compared among and between groups using one-way ANOVA with Tukey’s HSD. Correlation between mean MSOT oxygenated and deoxygenated hemoglobin intensity and colitis severity score for mice evaluated with both MSOT and colonoscopy at all three time points was assessed using Pearson correlation. Statistical analyses were performed using JMP software (JMP, SAS Institute Inc, Cary, NC). Differences were considered significant for p<0.05.
Table 1. Murine Endoscopic Index of Colitis Severity (MEICS) for Objective Quantification of Colonoscopic Findings.
Changes in colon thickness, vasculature, fibrin deposition, mucosal granularity, and stool consistence were all noted and assigned a score based on severity. These scores were summed to yield an overall colitis severity score. *From C Becker, MC Fantini, and MF Neurath. High resolution colonoscopy in live mice. Nature Protocols 1, 2900-2904 (2007)
| Murine endoscopic index of colitis severity (MEICS) | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | |
| Thickening of the colon | Transparent | Moderate | Marked | Non-transparent | 0–3 |
| Changes of the vascular pattern | Normal | Moderate | Marked | Bleeding | 0–3 |
| Fibrin visible | None | Little | Marked | Extreme | 0–3 |
| Granularity of the mucosal surface | None | Moderate | Marked | Extreme | 0–3 |
| Stool consistency | Normal + solid | Still shaped | Unshaped | Spread | 0–3 |
| Overall: 0–15 | |||||
Histology
After colonoscopy, mice were euthanized. The colon was resected in its entirety and flushed twice with PBS to evacuate residual stool. Colons were then bisected, fixed in 10% neutral buffered formalin, and embedded in paraffin. Sections were cut at 6 micron thickness and stained using H&E. Samples were blinded to the pathologist upon histologic analysis by a diagnostic pathologist at the University of Alabama, Birmingham Department of Pathology.
Results and Discussion
Mice treated with ETBF showed a mild increase in vascularity 2 days after bacterial inoculation (Figs 1 and 2), with an increase in mean signal intensity of oxygenated hemoglobin compared to untreated mice (1.150 vs. 2.716 MSOT a.u. compared to untreated mice; p=0.004). These findings were more prominent 7 days after inoculation, with increased mean signal intensity of oxygenated hemoglobin (1.150 vs. 2.716 vs. 3.422 MSOT a.u. for controls vs. 2 days post-ETBF vs. 7 days post-ETBF, p=0.0002) and the development of punctate lesions on the colonic surface (Fig. 1C). Deoxygenated hemoglobin signal obtained via MSOT remained similar in all mice evaluated regardless of treatment or timepoint.
Figure 1. Multispectral Optoacoustic Tomography Depicts Inflammatory Changes in Murine Colitis.
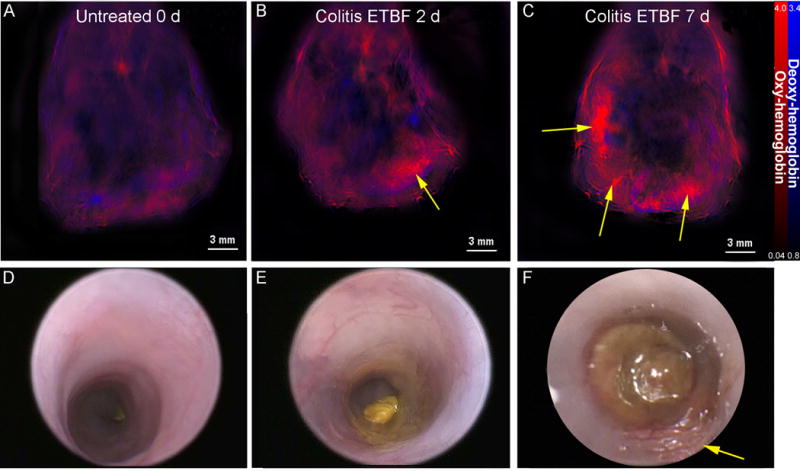
Wild-type C57B/6 mice were orally inoculated with either PBS alone (control) or ETBF. (A) depicts MSOT imaging of mice prior to ETBF treatment (untreated), while (B) and (C) represent images of mice 2 days and 7 days, respectively, after bacterial inoculation. Arrows indicate concentrated areas of oxy-hemoglobin corresponding to colitis (B–C). Findings on MSOT were compared to colonoscopic findings (D–F) for each group of mice. Arrow indicates an area of inflammation indicative of colitis (F).
Figure 2. Mean MSOT Signal Intensity for Oxy-hemoglobin Correlates with Colitis Severity Score.
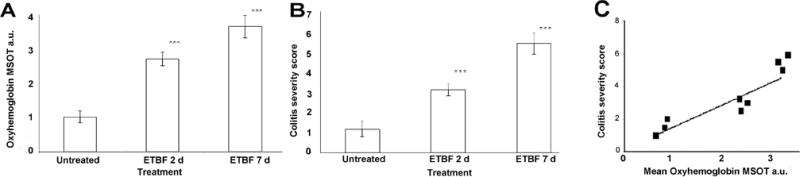
Region of intensity measurements were acquired for MSOT images were correlated to a colitis severity score determined from colonoscopy images. (A) demonstrates the mean signal intensity of oxy-hemoglobin for each group (control, 2d post ETBF inoculation, 7d post ETBF inoculation). *** indicates p<0.001. (B) demonstrates the average colitis severity score for each group. *** indicates p<0.001. Error bars represent standard deviation. (C) demonstrates the correlation between mean signal intensity of oxy-hemoglobin and mean colitis severity score for the 3 mice assessed using both MSOT and colonoscopy at all three timepoints (r=0.82, p=0.013)
The findings on MSOT correlated well with colonoscopic findings (r=0.82, p=0.013). Compared to untreated controls, mice at 2 days post ETBF inoculation demonstrated an increased colitis score (1.5 vs. 2.5), with mild colonic blunting and slightly deformable stool (Figs. 1D and 1E). At 7 days post-ETBF inoculation, mice displayed colonic blunting, vascular aneurysms, fibrin coats, patchy granularity, and deeply deformable stool (Fig. 1F), yielding an average colitis score of 5.5 (Figs 1 and 2B).
Additionally, findings on MSOT and colonoscopy corresponded well with blinded histologic analysis. Mice showed evidence of increasing inflammatory cell infiltrate and architectural distortion at both 2 days and 7 days after ETBF inoculation compared to controls (Fig. 3). A similar progression was also evident comparing mice 2 days and 7 days after ETBF inoculation. Findings were consistent across all mice in each group.
Figure 3.
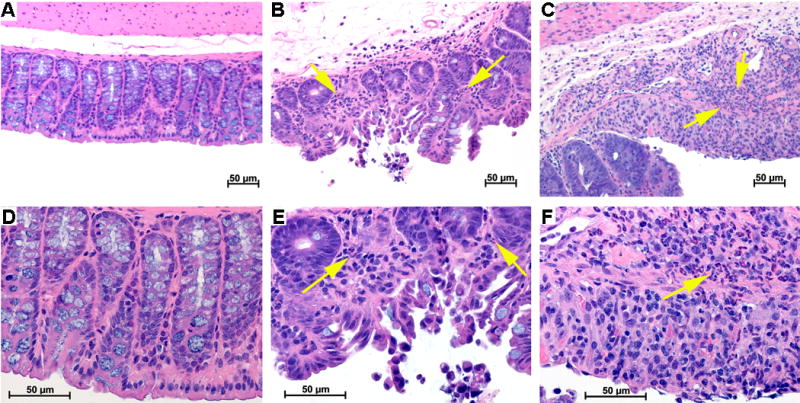
Histology Findings Demonstrate Inflammatory Changes Consistent with Colitis H&E analysis of mouse colon demonstrated no evidence of inflammatory cell infiltrate in control mice (A, D) and a progressively increasing polymorphonuclear leukocyte infiltrate 2 days (B, E) and 7 days (C, F) after ETBF inoculation as indicated by arrows. The histology images shown in C, F are from the mouse represented in Figure 1.
Our findings demonstrate that MSOT findings of hypervascularity and elevated levels of oxyhemoglobin are associated with inflammatory changes in the colon as well as inflammatory cell infiltrate evidenced on histology; this was associated with mild inflammatory changes on histology that were minimally detectable on colonoscopy in the same mice.
Clinically, MSOT offers multiple advantages over all current imaging and monitoring modalities including higher resolution without requiring exogenous contrast agents. Because quantification of oxygenated hemoglobin correlates with vascular changes seen on colonoscopy and inflammatory changes seen on histology, MSOT can provide an objective assessment tool that can be used to monitor the severity and progression. Additionally, given that polyposis and tumor growth are associated with local neo/hypervascularization, MSOT can be used to monitor IBD patients for tumorigenesis, with colonoscopy employed only if concerning features are identified on imaging studies.
Use of MSOT for diagnosis and monitoring of inflammatory bowel disease has the potential to significantly impact disease prognosis. The MSOT apparatus for use in human subjects consists of a handheld probe similar to bedside ultrasound (10, 13, 18). Improvements in depth of tissue penetration of optoacoustic imaging beyond 1 cm have been demonstrated in humans using the clinical MSOT system, Acuity, with up to 5 cm depth achieved (10, 18). Therefore, MSOT could be used to provide non-invasive screening for IBD in patients at high risk for developing the disease owing to factors such as family history and ethnicity, thereby enabling earlier detection of disease and commencement of therapy to prevent progression. Additionally, small changes detected on MSOT that may predict clinical worsening of disease (e.g. increased vascularity, development of small polyps) can prompt alteration of medical therapy and/or more timely colonoscopy and, if necessary, surgery, to prevent clinical worsening of disease or delayed detection of malignancy and risk of local invasion and/or metastasis. Conversely, intervals between invasive monitoring with colonoscopy can be increased in patients lacking significant changes in colonic and mesenteric vascularity and polyposis on MSOT over time. This would decrease monitoring cost as well as procedural risks and discomfort for IBD patients.
In summary, MSOT represents a non-invasive diagnostic modality that effectively identifies colitis in a murine model. Its diagnostic accuracy is at least equivalent to current standards of colonoscopy and tissue histology and surpasses that of conventional imaging modalities. With improvements in deep tissue penetration, these factors, together with MSOT’s detection of oxygenated and deoxygenated hemoglobin as endogenous contrast agents and correlation of signal intensity with colitis severity, would allow MSOT to serve as a viable modality for diagnosis and monitoring of patients with IBD for both progression of disease and development of neoplastic intestinal lesions.
Supplementary Material
Supplemental Figure 1: Experimental Schematic.
Supplemental Figure 2: MSOT detects increasing severity of colitis via increasing oxyhemoglobin.
Mice were imaged prior to ETBF treatment, 2 d post ETBF, and 7 d post ETBF treatment using MSOT. The images represent the additional 2 mice that were imaged longitudinally for 7 days are represented in the Top row and Bottom row. The third mouse images are located in Figure 1. Arrows indicate areas of focal colitis.
Acknowledgments
This work was supported by NIH grants R01CA205941 (LR McNally), R01EB020125 (LR McNally), R01CA100656 (NK Egilmez), and P30CA013148 (WE Grizzle).
Footnotes
Disclosures: The authors have nothing to disclose and do not declare any conflicts of interest.
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Tontini GE, Vecchi M, Pastorelli L, et al. Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World journal of gastroenterology. 2015;21:21–46. doi: 10.3748/wjg.v21.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointestinal cancer research : GCR. 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- 4.Ntziachristos V, Razansky D. Optical and opto-acoustic imaging. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2013;187:133–50. doi: 10.1007/978-3-642-10853-2_4. [DOI] [PubMed] [Google Scholar]
- 5.McNally LR, Mezera M, Morgan DE, et al. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clinical Cancer Research. 2016;22:3432–3429. doi: 10.1158/1078-0432.CCR-16-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutiani N, Kimbrough CW, Burton N, Morscher S, Egger ME, McMasters KM, El-Baz A, McNally L. Detection of microspheres in vivo using Multispectral Optoacoustic Tomography. Biotechnic & Histochemistry. doi: 10.1080/10520295.2016.1251611. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimbrough CW, Khanal A, Zeiderman M, et al. Targeting Acidity in Pancreatic Adenocarcinoma: Multispectral Optoacoustic Tomography Detects pH-Low Insertion Peptide Probes In Vivo. Clinical Cancer Research. 2015;21:4576–4585. doi: 10.1158/1078-0432.CCR-15-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton NC, Patel M, Morscher S, Driessen WH, Claussen J, Beziere N, Jetzfellner T, Taruttis A, Razansky D, Bednar B, Ntziachristos V. Multispectral opto-acoustic tomography (MSOT) of the brain and glioblastoma characterization. Neuroimage. 2013;65:522–528. doi: 10.1016/j.neuroimage.2012.09.053. [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Campbell J, Liu L, Mason RP, Lippert AR. In Vivo Chemiluminescent Imaging Agents for Nitroreductase and Tissue Oxygenation. Anal Chem. 2016;88:4995–5002. doi: 10.1021/acs.analchem.6b01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldner MJ, Knieling F, Egger C, et al. Multispectral Optoacoustic Tomography in Crohn’s Disease: Noninvasive Imaging of Disease Activity. Gastroenterology. 2016;151:238–240. doi: 10.1053/j.gastro.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 11.Razansky D, Buehler A, Ntziachristos V. Volumetric real-time multispectral optoacoustic tomography of biomarkers. Nature protocols. 2011;6:1121–1129. doi: 10.1038/nprot.2011.351. [DOI] [PubMed] [Google Scholar]
- 12.Ma R, Taruttis A, Ntziachristos V, Razansky D. Multispectral optoacoustic tomography (MSOT) scanner for whole-body small animal imaging. Optics express. 2009;17:21414–21426. doi: 10.1364/OE.17.021414. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26:375–385. doi: 10.1002/jmri.20969. [DOI] [PubMed] [Google Scholar]
- 14.Rabizadeh S, Rhee KJ, Wu S, et al. Enterotoxigenic bacteroides fragilis: a potential instigator of colitis. Inflammatory bowel diseases. 2007;13:1475–1483. doi: 10.1002/ibd.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee KJ, Wu S, Wu X, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infection and immunity. 2009;77:1708–1718. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nature protocols. 2006;1:2900–2904. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
- 17.Buehler A, Kacprowicz M, Taruttis A, Ntziachristos V. Real-time handheld multispectral optoacoustic imaging. Optics letters. 2013;38:1404–1406. doi: 10.1364/OL.38.001404. [DOI] [PubMed] [Google Scholar]
- 18.Stoffels I, Morscher S, Helfrich I, et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Science Translation Medicine. 2015;7:317ra199. doi: 10.1126/scitranslmed.aad1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Experimental Schematic.
Supplemental Figure 2: MSOT detects increasing severity of colitis via increasing oxyhemoglobin.
Mice were imaged prior to ETBF treatment, 2 d post ETBF, and 7 d post ETBF treatment using MSOT. The images represent the additional 2 mice that were imaged longitudinally for 7 days are represented in the Top row and Bottom row. The third mouse images are located in Figure 1. Arrows indicate areas of focal colitis.


