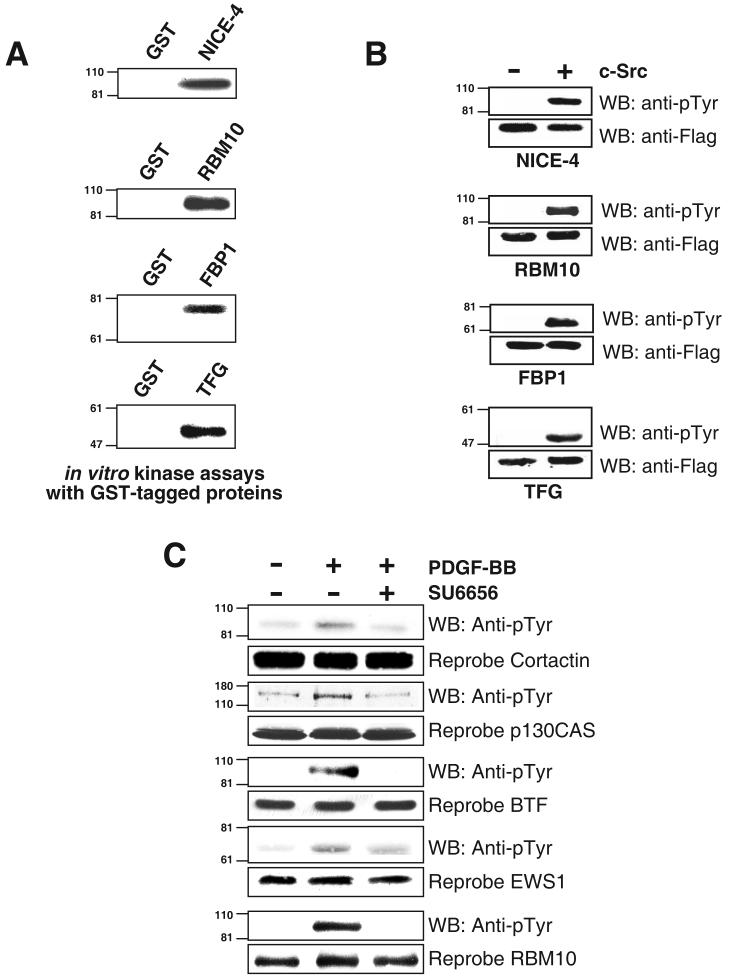
Figure 5.
Experimental validation of tyrosine phosphorylation of proteins obtained from c-Src kinase overexpression in 293T cells (A) In vitro Kinase assays using GST tagged proteins and c-Src using a rabbit reticulocyte in vitro transcription and translation system (B) 293T cells were co-transfected with genes of interest along with either empty vector PCMVtag4A or with c-Src. Culture media was changed 12 hours after transfection and cells were serum starved after serum starved for 12 hours and lysed 48 hours after transfection. Proteins were immunoprecipitated using anti-Flag antibodies and Western blotting was performed using phosphotyrosine antibodies and reprobed (C) Validation of a subset of proteins in PDGF signaling. NIH3T3 cells have been grown to confluence and serum starved for 12 hours followed by stimulation with PDGF-BB (100 ng/ml for 5 min) and PDGF stimulation after treatment with SU6656 (2 μM for 1 hour prior to lysis or stimulation) and cell lysates were subjected to immunoprecipitation using anti-phosphotyrosine antibodies and probed with respective antibodies, and re-probed in whole cell lysates.