Abstract
Many natural lectins have been reported to have antiviral activity. As some of these have been put forward as potential development candidates for preventing or treating viral infections, we have set out in this review to survey the literature on antiviral lectins. The review groups lectins by structural class and class of source organism we also detail their carbohydrate specificity and their reported antiviral activities. The review concludes with a brief discussion of several of the pertinent hurdles that heterologous proteins must clear to be useful clinical candidates and cites examples where such studies have been reported for antiviral lectins. Though the clearest path currently being followed is the use of antiviral lectins as anti-HIV microbicides via topical mucosal administration, some investigators have also found systemic efficacy against acute infections following subcutaneous administration.
I. Introduction
Lectins are proteins that bind to specific carbohydrate structures, but lack enzymatic activity. Many lectins inhibit the replication of viruses by interacting with viral envelope glycoproteins. Such antiviral lectins have been identified in bacteria, plants and marine algae. This review will discuss structural classes of lectins from various source organisms that have been investigated for their ability to block viral replication in vitro and to reduce the severity of illness and prevent death in virus-infected laboratory animals.
After briefly summarizing the structural basis for antiviral lectin binding and specificity, we review various antiviral lectins by source (bacteria, terrestrial or marine eukaryotic organisms) and structural class, discuss their selective carbohydrate binding profiles, and detail the published evidence of their antiviral activity. We then examine various challenges to both the topical and systemic use of lectins in the prevention or treatment of human viral infections, including their bioavailability, route of administration, toxicity and immunogenicity and their potential cost. In the concluding section, we discuss means by which the therapeutic utility of antiviral lectins might be improved.
II. Molecular mechanisms of antiviral lectins
A. Binding of lectins to carbohydrates
The molecular interactions between lectin and its carbohydrate substrate can be highly specific, recognizing both monomeric sugars as well as oligosaccharides formed as part of branched high-mannose or complex glycans (Fig. 1). Given the ubiquity of oligosaccharide post-translational modifications of proteins throughout all orders of life, lectins have evolved to play many roles in organismal biology including self-recognition, protein folding, and cell movement and adherence. As such, lectins have demonstrated potential for use in histologic studies to stain certain tissue types [1], or diagnostics in biosensors [2] as well as helping to understand and modulate cellular processes including host defense from infectious agents.
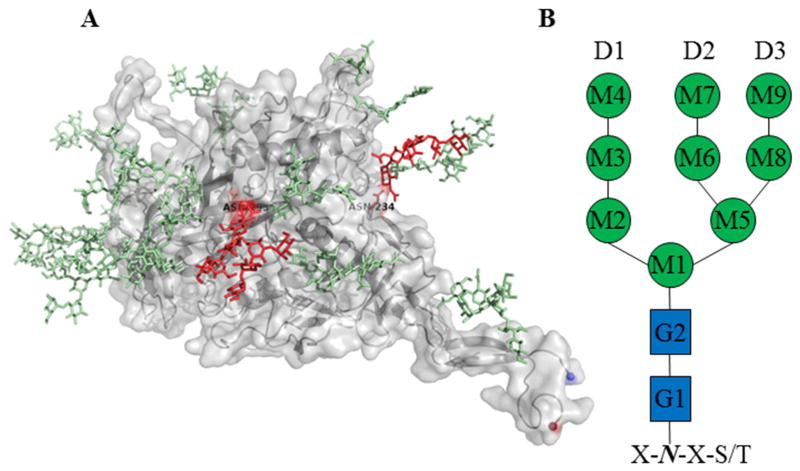
Figure 1. Structure of Monomeric gp120 Post-Translationally Modified with High-Mannose Glycans with a Representative High-Mannose Schematic.
A) The crystal structure of glycosylated gp120 monomer from HIV-1 clade G using the coordinates from PDBID: 5FYJ. gp120 is rendered in cartoon with surface in grey. The glycans are rendered in light green and red. The glycan positions rendered in red have been demonstrated to affect antiviral lectin activity of GRFT, CV-N, and SVN. B) Schematic of high-mannose for definition and discussion purposes. GlcNAc and mannose are represented as blue squares and green circles, respectively. The branches of the high-mannose structure are referred to as D1 for mannose 2–4, D2 for mannose 6 and 7 and D3 for mannose 8 and 9.
B. Specific mechanisms of antiviral lectin binding
A common route for viral recognition and entry utilizes glycosylated envelope proteins that have affinity for host cell-surface proteins [3]. The evolutionary development of viral glycosylation as a mechanism to both enhance viral uptake and evade host organism defenses has resulted in a co-evolution of lectins specific to non-self carbohydrate structures. The glycosylation of viral envelope glycoproteins is sequence driven, requiring spontaneous mutation and loss of oligosaccharide-attachment sites to avoid lectin recognition (Fig. 1) [4]. Such depletion of the glycocalyx surrounding envelope glycoproteins can have deleterious effects on viral fitness [5]. The continuing challenges in enabling broad spectrum viral suppression support the study of lectins as viral entry inhibitors to provide prophylactic and potentially therapeutic agents against viral infections.
Antiviral lectins interact predominantly with high-mannose glycan structures added as post-translational modifications to the envelope proteins of viruses [6–8]. The envelope proteins share sequence homology across enveloped viruses, adopt similar tertiary and quaternary structure, and perform equivalent functions (Fig. 2B) [9–11]. Using HIV as an example, the Env protein complex is composed of a transmembrane trimer of gp41 and extracellular trimer of gp120 (Fig. 2A) [12, 13]. Each of these envelope proteins contain N-linked oligosaccharide attachment sites (defined by the consensus amino acid sequence –NXS/T-) which, when glycosylated, assist in viral evasion of the host immune system (Figs. 1, 2C) [14]. The Env protein complex mediates attachment to and entry into target cells first through recognition of CD4+ initiating a cascade of conformation reorganization of the Env protein complex that ultimately leads to the fusion of the viral envelope with the host cellular membrane and infection (Fig. 2D) [15, 16]. Lectins are considered HIV entry inhibitors as they interact with the glycosylation moieties of the Env protein complex preventing the conformational rearrangements required for viral fusion [17].
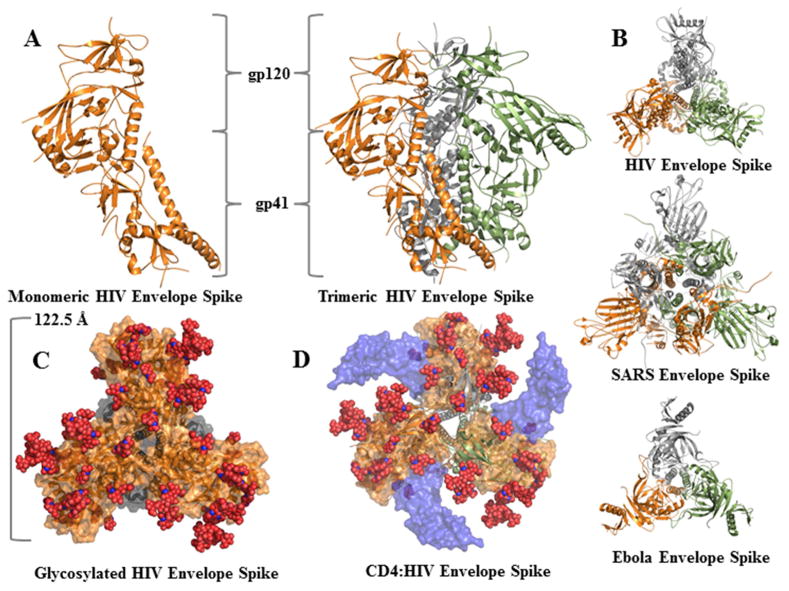
Figure 2. Viral Envelope Protein Complexes.
A) X-ray crystal structures of trimeric Env are rendered in ribbon with each monomer of gp41/120 shown in orange, grey, or green. B) The HIV (4TVP), SARS (5I08), and Ebola (5JQB) Env proteins are rendered in the same fashion, but viewed orthogonally along the three-fold symmetry axis to highlight structural conservation between viruses. C) Glycoproteins were rendered with the glycans as red spheres (4TVP). D) CD4:gp120 were modelled on the Env trimer to demonstrate what the recognition complex would look like (5CAY and 4TVP).
Antiviral lectins generally contain internal repeats within their primary sequences each comprising a carbohydrate recognition domain (CRD). The duplication of binding domains provides a mechanism for increased avidity for branched carbohydrate structures. The sequence identity of lectins ranges from ~10–100% across genera with the greatest variability localized to the loop regions that contribute to the CRD for some classes. Sequence homology is found predominantly in the core structural components of the lectin. Disulfide bonds are present in some antiviral lectin structures, but are not a prerequisite. Specifically, CV-N, SVN, and AH contain disulfide bonds that are within single domains and/or between domains. The lectins are also known to oligomerize, although it is also not a prerequisite. Some lectins form dimers, trimers, or form tetramers in solution, while others are seen as monomers (OAA, SVN, AH, and PCL) (Tables 2, 4, 6). Finally, certain members of the lectins discussed herein utilize strand exchange and domain swapping to stabilize the oligomeric state such as the C-terminal strand exchange seen in the β-prism type I and II folds and domain swap as seen in CV-N.
Table 2.
Structural Class and Glycan Specificity of Bacteria-derived Antiviral Lectins.
| Lectin | Mol. Wt. (kDa) | Oligomeric Status | Structure Class | Glycan Specificity | Glycoprotein Bound | References |
|---|---|---|---|---|---|---|
| Actinohivin | 12.5 | 1 | β-trefoil | α1,2mannotriose and Man9 (D1) | gp120 | [21, 28, 29] |
| Cyanovirin | 11 | 1 or 2 | CV-N-like | oligomannose (D1 D3) | gp120 | [6, 7, 31–33, 39, 40, 42–44, 49] |
| Microvirin | 14.2 | 1 | CV-N-like | α1,2mannotriose and Man9 (D1 D3) | gp120 | [23, 48, 50] |
| MVL | 13 | 2 | Unique | Man9GlcNAc2 (G1, G2, M1, M5, M8) | gp120 | [24, 39, 52] |
| Scytovirin | 9.7 | 1 | Unique | Oligomannose (M1, M5, M8, M9) | GP1 and E1/E2 | [25, 41, 54, 58] |
| OAA | 13.9 | 1 | OAAH | Manα(1,6)Mannose (M1 M5) | gp120 | [59–63] |
| PFA | 14 | 1 | OAAH | α(1,3),α(1,6) mannopentose (Core) | HIV | [63] |
| MBHA | 28 | 1 | OAAH | α(1,3),α(1,6) mannopentose (Core) | HIV | [63] |
| BOA | 29 | 1 | OAAH | α(1,3),α(1,6) mannopentose (Core) | Unk | [64] |
Unk - Unknown
Table 4.
Structural Class and Glycan Specificity of Plant-derived Antiviral Lectins.
| Lectin | Mol. Wt. (kDa) | Oligomeric Status | Structure Class | Glycan Specificity | Glycoprotein Bound | References |
|---|---|---|---|---|---|---|
| Jacalin | 16.5 | 4 | β-I | Galβ(1,3)GalNAc and Promiscuous (G1 or G2) | CD4* | [66–71] |
| BanLec (WT) | 30 | 2 | β-I | α(1,2), α (1,6) mannotetrose and Man9 (D3) | gp120 | [72–76] |
| GRFT (WT) | 12.7 | 2 | β-I | α(1,2)mannobiose (M4, 7, 9) | gp120 | [19, 80, 91, 157] |
| SCL | 13.1 | 4 | β-II | α(1,3:1,6)-mannotriose (Core) | HIV | [94, 95] |
| NPL | 12.0 | 4 | β-II | α1–3 or α1–6 linked mannoses (Core) | HIV | [97–100] |
| GNA | 12.5 | 4 | β-II | α1–3 or α1–6 linked mannoses (Core) | gp120 | [92, 99, 101–104] |
| HHA | 12.5 | 4 | β-II | Man5GlcNAc2 | gp120/gp140 | [96, 106, 107] |
| PCL | 12.0 | 2 | β-II | α1,3dimannoside (Core) | HIV | [108, 109] |
| ConA | 25 | 4 | ConA-Like | Man9GlcNAc2-type (D1 or D3) | gp120/gp41 | [110–113, 115, 116] |
| UDA | 8.5 | 1 | Hevein-Like | GlcNAc4 (G1 G2) | gp120/gp41 | [117, 119, 120] |
| MHL | 9.2 | Unk | Hevein-Like | GlcNAc (G1 G2) | gp120 | [124] |
| NICTABA | 19 | 2 | Unk | GlcNAc (G1 G2) | Gp41/120 | [126] |
Table 6.
Structural Class and Glycan Specificity of Marine-derived Antiviral Lectins.
| Lectin | Mol. Wt. (kDa) | Oligomeric Status | Structure Class | Specificity | Glycoprotein Bound | References |
|---|---|---|---|---|---|---|
| CVL | 30 | Unk | Unk. | Galactose | Unk | [127, 128] |
| SVL-1 | 15.5 | 4 | Unk. | Mannose | Unk | [129, 130] |
| SVL-2 | 12.7 | 4 | Unk. | GlcNAc (G1 G2) | Unk | [129, 130] |
| CGL | 18 | 1 | β-trefoil | GalNAc and Gal | Unk | [131–134] |
| DTL | 10.0 | 3 | Unk. | GlcNAc, chitobiose (G1 G2) | Unk | [130, 137, 158] |
| DTL-A | 14 | > | Unk. | GlcNAc and GalNAc (G1 G2) | Unk | [130, 137, 158] |
| GSL | 14.8 | Unk | Unk. | Mannose | gp120 | [138, 139] |
Unk - Unknown
Despite the similar function of specific recognition and tight binding to carbohydrates, lectins exhibit a wide range of tertiary structures (Fig. 3; Tables 2, 4, 6). These structural elements orient the CRD in space, yielding differences in the specific avidity of the three-dimensional oligosaccharide structures. Antiviral lectin folds discussed in this review include the type I β-prism as seen in Jacalin, GRFT, and BanLec, the type II β-prism as seen in the monocot derived lectins SCL, HHA, GNA, and NPL, the Ricin B chain-like β-trefoil exhibited by AH and CGL, cyanovirin lectin fold as seen in CV-N and microvirin, hevien-like as seen in UDA, as well as more unique folds including the β-barrel-like fold found in homologues of OAA, the 13 stranded antiparallel β-sheet of ConA, and the boomerang structure of MVL.
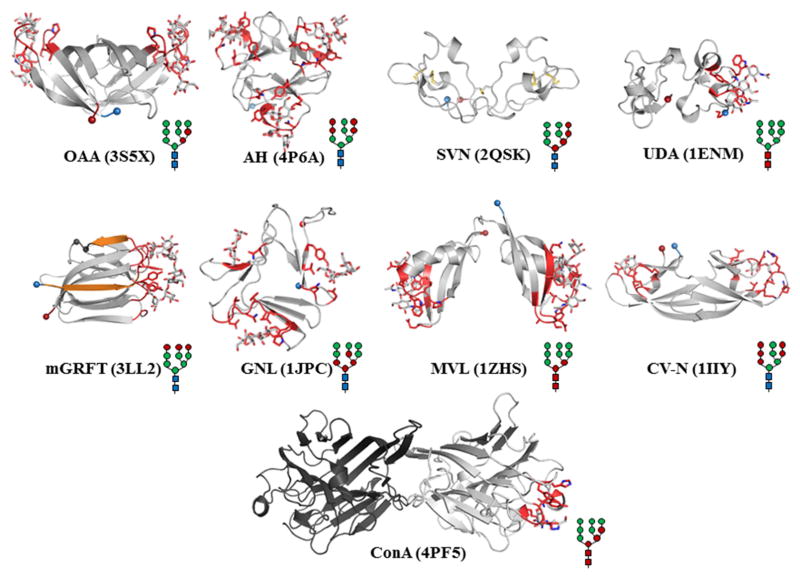
Figure 3. Representative Solution and Crystal Structures of Antiviral Lectins.
X-ray crystal structures with resolved saccharides in the CRD were selected when possible. NMR solution structures were selected when no crystal structures were available. The N and C termini are represented as spheres and colored blue and red, respectively. The overall folds are rendered in white with the residues that participate in CRD highlighted in red and side-chains shown as sticks. The saccharides are represented as sticks and colored according to atom. The high-mannose schematic displays interaction positions in red as identified from the literature. GRFT: The engineered monomeric form of the lectin is shown with the WT domain swapped portion in orange. The inserted GS residues are rendered as black spheres. ConA: The dimer is shown, with the monomer that bound saccharide as white and the apo monomer as black.
Lectins are generally classified based on the glycan recognition of their CRDs, such as specificity for particular sugars (i.e. mannose, glucose, N-acetyl galactosamine etc.). The structural orientation of the individual CRDs in lectins can then add increased levels of affinity for particular high-order sugar structures such as select oligosaccharides. Specifically, some antiviral lectins interact only with the high mannose oligosaccharide components M9, M7, and M4, some with entire glycan branches (D1, D2, or D3), and some interact with the core galactose containing chitobiose unit as well as D1 or D3 branches (Figs. 1, 3; Tables 2, 4, 6). The differences in CRD orientation can result in lectins demonstrating a substantial decrease in affinity when binding linear vs branched oligomannose structures, suggesting that the position, distance, and orientation of sugar units in space is important for lectin specificity.
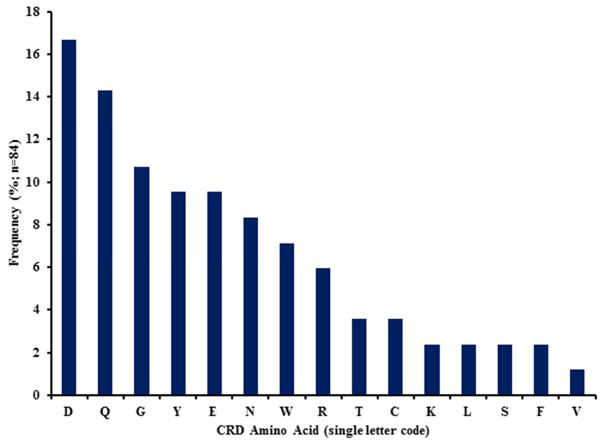
Analysis of the various antiviral lectin CRDs covered in this review reveals that many of the interactions are predominantly derived from hydrogen bonding between protein backbone and/or side chain atoms and oxygen atoms within the oligosaccharides. The Jacalin-like antiviral lectins utilize a trio of aromatic residues that provide the interior walls of the CRDs and in some instances the side chains of tyrosine and tryptophan may participate in direct interactions with the oligosaccharide. The lectins belonging to the OAAH family have a conserved consensus CRD sequence with residue side chains and backbone that participate in hydrogen bonding with the glycans. Analysis of the frequency of residues that participate in direct interactions with the oligosaccharides, either through back bone or side chain hydrogen bonding, reveals that aspartate and glutamate are most prevalent at 16.66% and 14.29%, respectively (Fig. 4). This is consistent with the types of lectin:glycan interactions identified through NMR titration and x-ray co-crystallization experiments. The less prevalent hydrophobic residues provide hydrogen bonding interactions specifically through backbone amide and carbonyl oxygen.
Figure 4. The Frequency of CRD Amino Acid Residues in Antiviral Lectins.
Lectins CRDs defined through NMR titration or x-ray co-crystallization experiments were analyzed for their frequency. A total of 84 residues from 17 lectins were used in the analysis. Aspartate and Glutamine were the most commonly found residues across all lectins reviewed. The hydrophobic residues participate in hydrogen bonding through their carbonyl and/or amide atoms. Tyrosine and tryptophan participate in both back bone and side chain interactions in some lectins. Note: If no experimental results are available for residues involved in glycan binding, then the lectin was excluded from the analysis.
The antiviral lectins detailed below all appear to work against viruses by a globally similar mechanism of binding to oligosaccharides decorating viral envelope glycoproteins and, via steric hindrance, inhibiting viral fusion and entry. This mechanism often requires multivalency of CRDs in the lectins themselves for adequate potency [18–20] and can often also rely on the cross-linking of oligosaccharides on Env by oligomeric lectins [17]. The biochemical basis of lectin antiviral activity therefore appears well established and has been detailed sufficiently to provide confidence that this mechanism of activity can indeed inhibit viral infection.
III. Antiviral lectins derived from prokaryotic organisms
Surprising structural diversity is seen in antiviral lectins derived from prokaryotic species, which includes the proteins actinohivin (AH) [21], cyanovirin-N (CV-N) [22], microvirin (MVN) [23], Microcystis viridis lectin (MVL) [24] and scytovirin (SVN) [25] amongst others (Fig. 3; Tables 1, 2). The protein folds in this group can be classified as β-trefoil, CV-N-like, OAAH-like, and “other”. The β-trefoil type fold is the only lectin fold that is shared between the prokaryotic and eukaryotic lectins discussed in this review. Furthermore, the prokaryotic derived lectins have broad specificity for specific oligosaccharide structures with members that bind to both high-mannose oligosaccharide core GlcNAc and mannose moieties.
Table 1.
Reported Antiviral Activities of Bacteria-derived Antiviral Lectins.
| Lectin | Source | Activity (nM) | Cytotoxicity | References |
|---|---|---|---|---|
| Actinohivin | Longisporum albid K97-0003 | HIV (2–110) HIV-1 (0.1–160), HIV-2 (2–33), SIV (11–160), HCV |
No | [26, 27, 30] |
| Cyanovirin | Nostoc ellipsosporum | (0.6–7.6), HSV-1 (61.82–205), Influenza A and B (0.46–118), Ebola (40–154), & MARV (6–25) | Yes | [31, 32, 34–41, 45, 46] |
| Microvirin | Microcystis aeruginosa PCC7806 | HIV (1.8–167) | Yes | [23, 48, 50] |
| MVL | Microcystis viridis NIES-102 | HIV & HCV (14–37) | Yes | [24, 39, 51, 52] |
| Scytovirin | Scytonema varium | HIV (0.3–187), Ebola (41), HCV (3.2–96), & MARV (260) | No | [8, 36, 55–58] |
| OAA | Oscillatoria agardhii NIES-204 | HIV (12) | Yes | [61–63] |
| PFA | Pseudomonas fluorescens | HIV (15) | Unk | [63] |
| MBHA | Myxococcus xanthus | HIV (12) | Unk | [63] |
| BOA | Burkholideria oklahomensis EO147 | HIV (10–24) | Unk | [64] |
Unk - Unknown
A. Actinohivin (AH) – β-Trefoil
Actinohivin (AH) was isolated from the actinomycete Longisporum albid strain K97-0003, a soil bacteria isolated in Japan [26]. The 114 residue protein has a molecular weight of ~12.5 kDa and contains 3 CRDs defined by internal repeats: R1 1–38, R2 39–77, and R3 78–114. AH is unusual among anti-HIV lectins in that it has a basic isoelectric point of 8.3. The protein is expressed as a proprotein with an N-terminal signal peptide that is subsequently cleaved revealing the mature 114 residue lectin. The lectin is active against HIV-1 and HIV-2 with IC50 values of 2–110 nM in the MAGI assay and inhibited cell-cell fusion with EC50 values ranging from 60–700 nM [27]. The x-ray crystal structures of AH reveal that the protein is organized into a Ricin B-chain-like β-trefoil with α1,2-mannotriose bound in each CRD located between the second and third β strands of the carbohydrate binding site [28]. Specifically, the side chains of D15, Y23, and N28 hydrogen bond with O3 and O4 of M2 of the trimannose ligand. The other sugar monomers are involved in secondary interactions mediated through water bridges and the binding interactions are conserved in all three CRDs. AH showed tight, specific binding to glycosylated gp120 (Kd = 5.3–23 nM), but no significant interaction with CD4 or co-receptors [29]. AH is thought to inhibit viral entry through binding to high-mannose oligosaccharides on gp120 with specificities similar to that of CV-N with no reported mitogenicity [29, 30]. Resistance studies revealed that HIV that became resistant to AH contained mutations that removed a mixture of 12 different N-glycosylation sites including 7 that typically bear high mannose oligosaccharides with up to 5 N-glycan deletion mutations necessary for a 20-fold reduction in AH potency [29].
B. Cyanovirin-like
1. Cyanovirin –N (CV-N)
Cyanovirin-N (CV-N) was isolated from the cyanobacterium Nostoc ellipsosporum cultivated by the University of Hawaii [31, 32]. This lectin has been the focus of much research as evidenced by more than 200 published papers. The protein contains 101 residues with a molecular weight of 11 kDa with monomeric and dimeric crystal forms and in solution. The protein adopts a unique fold comprised of a tandem repeat, defined by R1 1–50 and R2 51–101 (31.1% sequence identity), adopting a three-stranded β-sheet with β-hairpins and has exhibited a domain-swapped dimer in various crystal and solution structures [7, 33]. The structure of CV-N co-crystallized with oligomannose-9 revealed extensive interactions with the outer branches of high-mannose glycan. Specifically, K3 and E101 side chains hydrogen bond to O2 and O3 of M4, M3 interacts with the carbonyl of G2 and K53, the amide of N93, and the hydroxyl of T7, and mannose 2 hydrogen bonding to the carbonyl of N93 (O3 and O2), the carbonyl and side chain of E23 (O4 and O6, respectively), and the amide of D95 (O3).
Extensive antiviral research has shown that CV-N is active against HIV-1 (0.1–160 nM), HIV-2 (2–33 nM), SIV (11–160 nM), HCV (0.6–7.6 nM), HSV-1 (61.82–205 nM), Influenza A and B (0.46–118 nM), EBOVZ (40–154 nM), and MARV (6–25) [31, 34–41]. Most research was carried out against HIV-1 revealing IC50 values of 0.1–160 nM depending on HIV strain and cell-type as well as when the lectin was administered in the challenge experiment. As with other lectins discussed in this review, CV-N has been shown to interact specifically with gp120, or homologous, high-mannose oligosaccharides (Ka for man(1,2)man 7.2×106 M−1 and 6.8×105 M−1, for the two CRDs) and thought of as a viral entry inhibitor [6, 39, 40, 42–44]. Neutralization of CV-N antiviral activity through titrations with increasing concentrations of gp120 fit best to a two-independent site model supporting 2:1 stoichiometry of CV-N:gp120 and revealed an IC50 of 17 nM [44]. CV-N completely inhibited Env-induced, CD4-dependent cell-cell fusion at 100 nM and gp120-derived mAb mapping studies indicated that CV-N does not affect the gp120:CD4 interaction [44].
a. In vivo Preventative Microbicide
Importantly, CV-N produced no cytotoxic effects when applied as a 0.5–2% CV-N topical gel rectally or vaginally in male and female macaques challenged with SIV/HIV-1 chimeric virus (SHIV89.6P) but was effective in preventing SHIV infection [45, 46]. The evaluation of CV-N for topical HIV prevention is continuing with the recently-funded study NIH RePORT in which constitutively expressed CV-N in Lactobacillus jensenii will enter Phase I clinical trials in the form of MucoCept, a quick dissolving vaginal suppository containing the recombinant live biotherapeutic product [47].
b. In vivo Parenteral Therapeutic
Likewise, subcutaneous injection of 5 mg/kg CV-N in a lethal mouse model of Ebola-Z infection in mice resulted in no immunologic response with a decrease in mortality and viral titer [37]. Taken together, these results indicate that CV-N is an antiviral lectin that has shown potential utility in preventing viral infections.
2. Microvirin (MVN)
Microvirin (MVN) was isolated from the cyanobacterium Microcystis aeruginosa PCC7806 [48]. The monomeric lectin has a reported molecular weight of 14.2 kDa for the 108 residues. Microvirin adopts a Cyanovirin-like fold with an RMSD of 1.77 Å over 88 α-Carbons, which is not surprising given the 31.5% sequence identity. Examination of the CRDs between the CV-N (solution: 1IIY) and MVN (solution: 2YHH) structures reveals similar mannose poses with a conserved binding pocket and homologous sugar:protein interactions with Q81 side chain carbonyl hydrogen bonding to O6 of a mannose monomer and more extensive interaction with the second monomer through hydrogen bonding with the carbonyls of Q54 and N55 (O3 and O4, respectively) and E56 carboxylate interacting with O6 of the same monomer [23, 49].
Microvirin was tested against HIV-1 in various cell types including X4, X4+R5, as well as clinical isolate groups M and O, and Env pseudotyped X4, R5, and X4/R5 in TZM-bl cells with a range of IC50 values of 1.8–167 nM [23, 50]. MVN was not active against clinical isolate group O in PBMC (>350 nM) or HIV-2 (>330 nM) at the tested concentrations. The development of HIV-1: MVN-resistance was explored with the NL4.3 virus in MT-4 cells at dilute concentrations of MVN revealing 4 mutations in gp120 of the resistant strain (residues T294, T341, N386, and N392) [50]. The lectin specifically binds to oligosaccharides of viral envelope glycoproteins with preference for high-mannose glycans containing terminal α(1,2)-mannose disaccharides and the highest signals reported for Man(9), the D1 arm of Man(9), and the D3 arm of Man(9) as determined from a carbohydrate microarray [48]. MVN inhibits syncytium formation as well as DC-SIGN-mediated viral binding to CD4+ cells [50]. Even though MVN displayed a less pronounced mitogenic response when compared to CV-N in freshly isolated PBMCs from two separate donors, significant stimulatory effects on the production of cytokines IL-1β, IL-6, IL-10, G-CSF, IFN-γ, MIP-1α, and TNF-α were observed [50]. The protein also inhibited syncytium formation in HIV-1 infected HUT-78 T cells and uninfected CD4+ SupT1 cells and, like CV-N, prevented the binding of the anti-HIV mAb 2G12 high-mannose gp120 (albeit at 10 fold lower inhibition) [49]. MVN acts synergistically with both 2G12 and CV-N [23]. These results support that microvirin is a broad HIV-1 entry inhibitor effective against many co-receptor types as well as the lab clinical isolate group M HIV-1 in PBMC.
C. Microcystis viridis lectin (MVL)
MVL was isolated from the cyanobacterium Microcystis viridis NIES-102 strain [51]. The homodimeric lectin is 13 kDa consisting of 113 residues, and contains 2 internal repeats defined by residues 2–55 and 61–114 that have 45% sequence identity [24]. The apo and holo crystal structures (PDBID: 1ZHS and 1ZHQ) revealed a 2 domain organization where each domain contains a distorted 3-stranded antiparallel β-sheet that wraps around a 15 residue α-helix effectively forming a cleft at the distal end of the domain [24]. The lectin forms a dimer in crystal and solution with extensive interactions burying ~3900Å2 of solvent accessible surface. Each monomer interacts with both N- and C-terminal domains in the dimer. This domain organization results in a cleft that contains the CRD, which accommodates pentasaccharides through direct interactions with 4 sugar monomers.
MVL has been reported to have anti-HIV-1 and HCV activities at 30–37 nM and 14–34.3 nM, respectively [24, 39, 52]. Pre-incubation of HCV with Man9GlcNAc2 prevented viral inhibition by MVL in a dose dependent manner, which is consistent with MVL inhibiting enveloped viruses through the interaction with glycans of glycoproteins [39]. The binding affinities of MVL to recombinant HCV E1E2 glycoprotein were measured using biolayer interferometry revealing a KD of 1.8×10−4 M [39]. MVL is thought to inhibit viral entry through binding high-mannose glycans on gp120, such as Man6GlcNAc2 with sub-micromolar affinity (2.22×10−7 M) [52]. Titration of MVL and gp120 supported that 2 molecules of MVL interact with a gp120 trimer for effective inhibition of virus.
Initially discovered for its glycan binding interactions, MVL was also reported as having enzymatic activity representing a novel bifunctional mode to the lectin family [53]. Specifically, one of the CRDs is capable of catalyzing the enzymatic cleavage of chitotriose to GlcNAc. MVL binds GlcNAc3 and GlcNAc4 with KDs of 27 and 60 μM, respectively, which is 10 to 20 times weaker than Man3GlcNAc2. The authors discovered that D75 is the primary residue enabling the enzymatic cleavage to proceed. Although lectins are defined as not having enzymatic activity, MVL represents the only bifunctional lectin discussed in this review.
D. Scytovirin (SVN)
SVN was isolated from the cyanobacterium Scytonema varium by Bokesch et al. [8]. The monomeric lectin is 9.7 kDa and comprised of 95 residues organized as 2 internal repeats with 75% sequence identity. The overall fold of SVN is loose in secondary structure containing 10 cysteines with 2 inter-domain disulfide bridges per internal repeat and 1 intra-domain disulfide linkage per molecule resulting in 5 total cystines [25, 54]. The 2 CRDs have different affinities for tetramannose with Scytovirin Domain 1 (SD1) having higher affinity. Research efforts have focused on generating SD1 and SD2 monomers revealing that the bulk of SVN activity derives from SD1 specifically [55]. Of the three structures present in the Protein Databank (PDB), none of them contain oligomannose in the CRD. However, 15N-HSQC oligosaccharide titration studies identified chemical shift perturbation for SD1 residues W8, N9, F37, C38, and Q39 and SD2 residues W56, D57, E58, F85, C86, and Q87. It was rationalized that the decreased affinity for SD2 to oligomoannose results from the increased electronegativity from the residue substitution of asparagine to aspartate.
The monomeric lectin was tested against a variety of viruses including HIV-1, Ebola, HCV, Marburg virus (MARV) with a range of IC50 from 0.3–395.5 nM [8, 36, 55–58]. The sub-nanomolar activities were seen with HIV-1 including co-receptor variants X4 as well as X4 and R5. SVN interacts with envelope protein high-mannose glycans containing α(1–2),α(1–2),α(1–6)-tetramannose with a KD of 30 μM for SD1 and 160 μM for SD2 [54]. Western blot analysis supported that SVN interacts with glycosylated ZEBOV GP1 [58]. Likewise, SVN was also shown to interact specifically with the high-mannose glycans on the envelope glycoproteins E1 and E2 in HCV [41]. These findings support that SVN inhibits viral entry by interacting with the glycans on viral envelop proteins.
a. In vivo Parenteral Therapeutic
SVN was subcutaneously injected into BALB/c mice infected with Ebola (ZEBOV) or MARV at 10, 20 or 30 mg/kg/day (in four doses) revealing no signs of cytotoxicity with a 90% survival rate highlighting the therapeutic potential of the lectin [58]. The IC50 of SVN for ZEBOV and MARV was 41 and 260 nM, respectively. The injected SVN was bioavailable in BALB/c mice showing marked decreases in baseline levels within 4 hours of injection, thus requiring the 4 injections per day to maintain effective antiviral serum concentrations, which greatly complicated BSL-4 containment efficacy studies. The survival rate past 20 days was 70–90% for SVN treatments −1 hour, +1 hour, and +1 day after viral challenge indicating the efficacy of systemic treatment in a mammalian system. Despite the short half-life of injected SVN, the potency against a range of sensitive viruses and the absence of reported cytotoxicities earns this particular antiviral lectin a high potential therapeutic status. Though SVN displayed good efficacy in protecting mice against ZEBOV, the challenges associated with its short half-life make further development of this agent dependent on modifications of the protein which could extend its serum half-life (i.e PEGylation-modification with polyethylene glycol).
E. Oscillatoria agardhii Agglutinin Homologues (OAAH)
The crystal structures of OAAH are each comprised of 10 β-strands that fold into a β-barrel-like architecture with the mannose binding sites localized to the loops between β1- β2 and β9- β10 for CRD1 and β4- β5 and β6- β7 for CRD2 and NMR solution structures in bound and free state revealed dynamic loops at the CRD [59, 60]. The CRDs of Oscillatoria agardhii Agglutinin Homologes (OAAH) antiviral lectins have consensus sequences that include three regions specifically defined as (using OAA numbering) residues 7–11 (NQWGG), 93–99 (GXRX(1,2)QXV), and 122–128 (GEGPIGF). These regions are loops located between β-strands and are present in each of the antiviral lectins mentioned below. The homologs have high sequence identity of 63–79% between each internal repeat with some members containing 4 repeats. The OAAH lectins discussed in this review each have IC50s of ~10–15 nM in TZM-bl cells and interact specifically with core α(1,3),α(1,6)-mannoses.
1. Oscillatoria agardhii Agglutinin (OAA)
Oscillatoria agardhii
Agglutinin (OAA) was isolated from the cyanobacterium Oscillatoria agardhii strain NIES-204 [61]. The lectin has a molecular weight of 13.9 kDa and consists of 133 residues that make up two tandem repeats defined by residues 1–67 and 68–133 with 76.1% sequence identity [62]. Examination of the apo and holo crystal structures revealed a dimer of the consensus OAAH-lectin fold with little conformation changes upon carbohydrate binding. NMR titration of man-9 and OAA revealed that the two CRD have different affinities for the mannoses, with CRD2 having a higher affinity with an estimated KD of 10 uM [60].
OAA was reported to have anti-HIV-1activities with EC50 of 44.5 nM using the co-receptor strain Lab X4 and an IC50 of 12 nM in TZM-bl cells [61–63]. The lectin specifically interacts with high-mannose-type N-glycan α(1,6)-mannose disaccharides as revealed from NMR titration experiments. The dissociation constant for OAA to a heptasaccharide high-mannose type N-glycan was determined through centrifugal ultrafiltration-HPLC with fluorescently labeled oligosaccharides (4.15×10−9 M) and revealed that the lectin possesses two carbohydrate binding sites per molecule, which is consistent with the tandem repeat in the primary sequence. OAA also interacts with recombinant glycoprotein gp120 with a KD of 2.54×10−12 M as determined from surface plasmon resonance. The tremendous affinity for heptasaccharide mannose and glycoprotein with only modest EC50 against HIV-1 highlights an important aspect of lectin based antiviral mechanism of action – namely that the strong interaction with the substrate glycan does not necessarily correlate with the effectiveness of viral inhibition (at least in the case of OAA).
2. Pseudomonas fluorescens Agglutinin (PFA)
Pseudomonas fluorescens
Agglutinin (PFA) was identified from a sequence search for OAA homologues [63]. The hypothetical protein sequence was 63% identical and 73% similar to OAA primary sequence over the 2 tandem repeats in PFA. The lectin was selected for recombinant expression in E. coli DE3 for biochemical, biological, and structural characterization. PFA is a 14 kDa protein comprised of 133 residues with 2 sequence repeats and the crystal structure revealed a tertiary structure homologous to OAAH-lectin fold (4FBO).
The lectin was active against HIV-1 in TZM-bl cells with an IC50 of 15 nM. NMR titration experiments with PFA and α(1,3),α(1,6)-mannopentose resulted in fully occupied CRD1 and CRD2 at 1:3 protein:sugar molar ratios [63]. Consistent with OAA, the resonances of CRD1 are perturbed earlier in the titration experiment than the residues in CRD2, supporting a difference in affinity for the mannopentose substrate between the two binding domains.
3. Myxococcus xanthus Hemagglutinin (MBHA)
Similar to PFA, Myxococcus xanthus Hemeagglutinin (MBHA) was identified from a sequence search for OAA homologues [63]. The hypothetical protein sequence was 63% identical and 79% similar to OAA primary sequence over the 4 tandem repeats of MBHA. The lectin was selected for recombinant expression in E. coli DE3 for biochemical, biological, and structural characterization. MBHA is a 28 kDa protein consisting of 267 residues with 4 sequence repeats. The lectin is monomeric in solution with x-ray crystal structures revealing an OAAH-lectin fold (4FBR). Interestingly, the 3 and 4 sequence repeat orientation is flipped 180 ° with respect to the 1 and 2 sequence repeats. This is in contrast to the orientation of the two proteins in crystallographic packing of PFA (4FBO).
The lectin was active against HIV-1 in TZM-bl cells with an IC50 of 12 nM [63]. NMR titration experiments with MBHA and α(1,3),α(1,6)-mannopentose resulted in fully occupied CRD1, CRD2, CRD3, and CRD4 at 1:6 molar ratio. Similar to other OAAH-lectin members, the resonances attributed to CRD3, and CRD4 are perturbed earlier in the NMR titration experiment than the residues in CRD1 and CRD2, suggesting a difference in affinity for the mannopentose substrate between the four binding sites. These results indicate the initial interaction between glycan and lectin takes place with CRD3 and CRD4 followed by subsequent binding to CRD1 and CRD2 and suggest that primary oligosaccharide recognition is dictated by the CRD3 and CRD4 binding domains.
4. Burkholderia oklahomensis Agglutinin (BOA)
Burkholderia oklahomensis
Agglutinin (BOA) was isolated from the proteobacterium Burkholideria oklahomensis EO147 [64]. The 276 residue protein contains an N-terminal 10 residue tail that is not present in the other OAAH members. Like MBHA, the primary sequence of BOA is comprised of 4 tandem repeats with the x-ray crystal structures confirming a dimeric OAAH-lectin fold with four CRDs. The sequence repeats share 66% sequence identity over 126 residues. Superposition of the individual monomers onto the canonical OAA structure resulted in a Cα RMSD ~0.6 Å. Furthermore, the conformations of CRD bound with oligomannose were conserved with only small conformational heterogeneity observed in loops.
Similar to each of the OAAH members, BOA was active against HIV-1 in TZM-bl cells with an IC50 range of 10–14 nM [64]. Dissociation constants were estimated using 1H–15N HSQC titration experiments with BOA and α(1,3),α(1,6)-mannopentose and reported as 20–67 μM for all four CRDs bound. Due to the nature of the experiment, individual CRD KDs could not be assigned, but the averaged IC50 is comparable to other OAAH members.
IV. Antiviral lectins obtained from plants
The plant eukaryotic antiviral lectins also have a range of structural diversity that can be classified as β-prism type I, β-prism type II, concanavalin-like, and hevein-like folds (Fig. 3; Tables 3 and 4). The eukaryotic antiviral lectins contain tandem repeats within the primary sequence, exist in monomeric and higher multimeric states, and, with the exception of the hevien-like class, are comprised largely of β-sheets. The eukaryotic lectins also have a range of glycan binding specificities that include GlcNAc only, GlcNAc:Man, core mannose, high-mannose, with some GalNAc specificities as well (Table 4).
Table 3.
Reported Antiviral Activities of Plant-derived Antiviral Lectins.
| Lectin | Source | Activity (nM unless otherwise noted) | Cytotoxicity | References |
|---|---|---|---|---|
| Jacalin | Artocarpus integrifolia | HIV (60–600) | No | [65, 69] |
| BanLec (WT) | Musa acuminate | HIV, HCV, H1N1, & H3N2 (0.28–20.8 nM) | Yes | [72, 75–78] |
| GRFT (WT) | Griffithsia sp. | HIV-1 (0.02–56), HIV-2 (0.11–0.24), HCV (0.3–14.1), HSV-2 (0.181–11.26 μM), SARs-CoV (0.048–960), various avian CoV subtypes (0.032–0.57), BCoV (0.057), IBV (0.032), MHV (0.23), PCoV (0.57), HCoV and mutants (0.16), JEV (20), SIV (0.95–1.24), & SHIV (0.02–0.83) | No | [36, 57, 79, 81–88] |
| SCL | Scilla campanulata | HIV (4.6–8 μM) | Unk | [94, 95] |
| NPL | Narcissus pseudonarcissus | HIV & SIV (0.7–7.3 μg/mL) HSV, Rabies, & Rubella (> 1 μM) | Unk | [96, 98–100] |
| GNA | Galanthus nivalis | HIV-1 (0.33–4.7 μg/mL), HIV-2 (0.12–0.25 μg/mL), SIV (2.7 μg/mL), & FIV (0.09 μg/mL) | No | [99, 101, 102, 105] |
| HHA | Hippeastrum hybrid | HIV-1 (0.3–3.2 μg/mL), HIV-2 (0.16–0.18 μg/mL) SIV (0.60 μg/mL), & FIV (0.16 μg/mL) | No | [96, 105] |
| PCL | Polygonatum cyrtonema Hau | HIV (0.05–0.1 μg/mL) | Unk | [108] |
| ConA | Canavalia ensiformis | HIV & HSV (88 and 98) | Yes | [35, 110, 114] |
| UDA | Urtica dioica | HIV, CMV, RSV, H1N1, & SARS-CoV (0.1–1 μM) | Yes | [118, 121–123, 156] |
| MHL | Myrianthus holstii | HIV-1 (150) | Unk | [124] |
| NICTABA | Nicotiana tabacum | HIV-1/2 (5–30), HSV (53–263), Influenza A/B (11–32), & RSV (105) | No | [126] |
Unk - Unknown
A. β-Prism Type I
1. Jacalin
The lectin, Jacalin, was isolated from jackfruit seeds (Artocarpus integrifolia) [65]. The lectin exists as a homotetramer in solution with a monomeric molecular weight of 16.5 kDa. The most important contribution of this lectin to the antiviral lectins discussed in this review is the wealth of structural information published to date [66–68]. Twelve PDB entries represent some of the first structures that described the type I β-prism fold, commonly referred to as the Jacalin-like lectin fold. The type I β-prism fold has three-fold symmetry with each domain comprised of a four-stranded Greek key motif. The protein is post-translationally modified through proteolytic cleavage of the first 18 residues in the N-terminus resulting in the heavy α Jacalin chain and the light 18 residue peptide β chain. In the 1JAC crystal structure, Gly1 of the α-chain forms a portion of the CRD with G1 carbonyl H-bonding with the O3 and O4 hydroxyls of methyl-α-galactose [67]. The CRD is largely comprised of hydrophobic aromatic residues: F47, Y78, Y122, and W123 exhibiting hydrogen bonding interactions between galactose and Y122, W123, and G1 amides and D125 carboxylate..
Jacalin exhibited sub-micromolar anti-HIV activity with an EC50 of 60–600 nM depending on the assay cell type [69]. Jacalin is distinct from the other antiviral lectins discussed in this review in that the mechanism of action most likely derives not from the interaction of lectin and glycoprotein gp120, but rather the interaction with glycans that decorate CD4 on the host cell surface [69–71]. It was initially thought that Jacalin specifically bound Galβ(1,3)GalNAc, but subsequent SPR and crystallographic studies revealed that Jacalin is promiscuous in saccharide binding with a CRD that accommodates GalNAc, GlcNAc, Neu5Ac, MurNAc, and 1-O-methyl derivatives of glucose, mannose, and galactose (albeit at a much higher concentration) [66, 70, 71]. Furthermore, a 14 residue peptide derived from Jacalin is homologous with a portion of gp120 sequence exhibiting mild inhibitory effects on HIV (at ~318 uM) [69]. Jacalin is the only lectin discussed in this review that is thought to have a preference of CD4 glycans over gp120.
2. BanLec
The BanLec lectin was isolated from banana, Musa acuminate, and exists as a homodimer with a molecular weight of 30 kDa [72]. The lectin exhibits a Jacalin-like lectin fold with an RMSD of 0.934 Å over 92 α-carbons when superposed with the Jacalin structure (1JAC) (Fig. 5) [73, 74]. Examination of the CRDs in the x-ray crystal structures 1X1V and 1JAC for BanLec and Jacalin, respectively, revealed a similar pose for o-methyl-mannose as well as the overall organization of the CRD loops. More specifically, mannose interacts with the amides of D35, V36, and G60 as well as the carboxylate of D38. However, BanLec does not exhibit domain swapping or the proteolytic cleavage of the N-terminal 18 residues as seen in Jacalin.
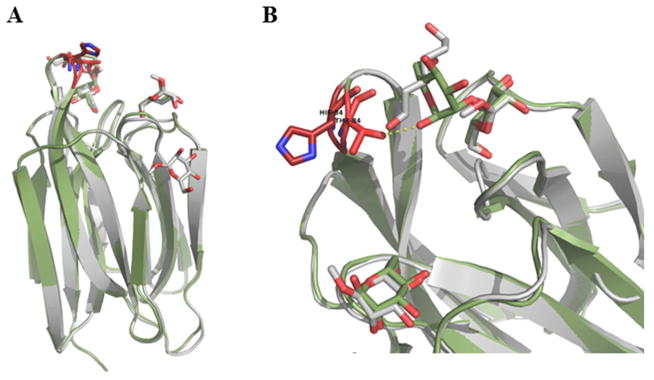
Figure 5. Superposition of BanLec WT and H84T Mutant Structures.
A) The WT (grey) and H84T mutant (green) structures were aligned with a root mean squared deviation of 0.442 Å over 138 C-α positions. Mannose and dimannose are rendered as sticks. Residue 84 is rendered as sticks in the two structures and colored red. B) Close up of the loop containing residue 84 and BanLec CRD. Despite the decrease in mitogenicity, the overall fold of the protein is conserved with only the loop containing residue 84 repositioned with the C-α 1.4 Å closer and C-β 2.1 Å closer to the CRD in the mutant structure. This repositions results in an additional Hydrogen bond between 84T and dimannose hydroxyl.
The lectin contains 2 CRDs per monomer and is potently active against HIV-1 in TZM-bl indicator cells with a range of IC50 values of 0.48–2.06 nM and 0.28–2.3 nM in pseudotyped HIV-1 [74, 75]. BanLec interacts directly with HIV-1 glycoprotein high-mannose gp120, which resulted in blocked viral entry and decreased levels of the strong-stop product of early viral transcription [75]. Antiviral activities of rBanLec H84T were reported with EC50 values of 8.8–20.8 nM across various HCV genotypes and EC50 values of 1–4 μg/ml and 0.06–0.1 μg/ml for H1N1 and H3N2 virus in MDCK cells, respectively [76].
a. in vivo Preventative Microbicide
Intranasal (IN) administration of rBanLec H84T 4 hr post IN infection (and daily for 5 days) of mouse model with H1N1effectively blocked viral infection with approximately 75% survival rate with the low dose of 0.03 mg. Wild type BanLec and rBanLec are potent T-cell mitogens [76, 77]. rBanLec had an immunostimulatory effect and formed complexes with superficial mucus of the jejunum [78]. rBanLec is also stable in mouse gastrointestinal tract, in vivo, where the lectin interacts with glycosyl groups of the mucosal surface.
b. Efforts to Reduce Mitogenicity
Because the lectin exhibited a strong T-cell mitogenic response, research was conducted to mutate the protein to reduce mitogenicity while preserving the virucidal activity [76]. In effect, the mutation H84T resulted in a substantial decrease in mitogencity while maintaining the antiviral effects [76]. Interestingly, the mutation is positioned near the CRD yet had little effect on the virucidal activity and did not substantially alter the mannose poses in crystallo (4PIT). Alignment of the WT and H84T BanLec structures results in a root-mean-squared deviation of 0.442 Å over 138 C-α positions with only slight perturbations in the backbone for residue 84 indicating strong structural conservation for the remaining protein backbone (Fig. 5). The repositioned loop results in residue 84 C-α 1.4 Å closer and C-β 2.1 Å closer to the CRD in the mutant structure allowing an additional hydrogen bond between 84T and dimannose hydroxyl. This methodology, which reduces mitogenicity and retains structural integrity, provides a route for the potential development of antiviral lectin-based therapeutics. Due to the potency against HIV and the low mitogenicity of the recombinant H84T mutant, BanLec is of potential therapeutic importance with respect to many of the antiviral lectins discussed in this review.
3. Griffithsin
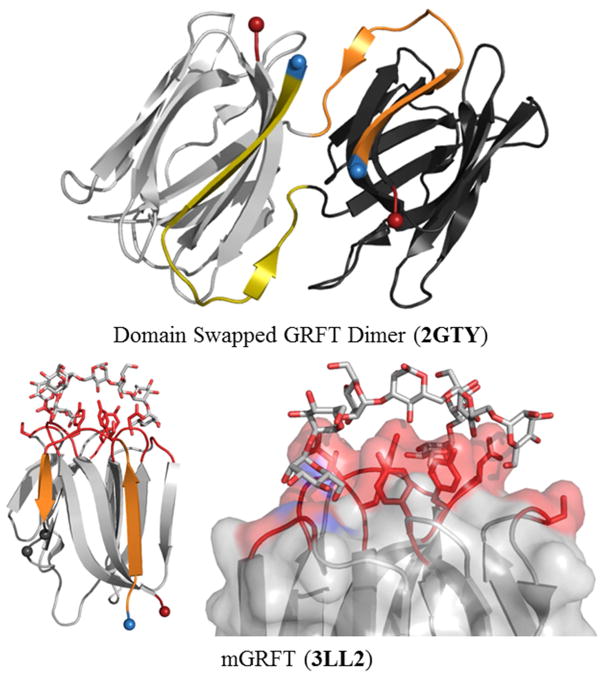
Griffithsin (GRFT) was isolated from the aqueous extract of the red algae Griffithsia sp. collected off the eastern shore of Chatham Island, New Zealand [79]. The lectin is 12.7 kDa, comprised of 121 residues, and exists as a homodimer in solution. Extensive biological, structural, and biophysical research has been conducted on this extremely potent antiviral. GRFT adopts a Jacalin-like β-prism type I fold with an RMSD of 1.53 Å over 57 α-carbons and contains a N-terminal 2 β-strand exchange to form a stable dimer (using PDBIDs: 2GTY unliganded GRFT and 1JAC Jacalin) (Fig. 6) [80]. Like other type I β-prism fold lectins, the griffithsin CRD contains three aromatic residues: tyrosine residues 27, 68, and 110, which form the interior of the binding site while the side chains of D112, Y110, and the amides of G12, D109, G108, Y110 hydrogen bond with mannose oxygens as seen in the crystal structure in complex with α(1,6)-mannobiose. More extensive interactions are seen in the co-crystal structure of monomeric GRFT and nonamannoside terminal mannose units (M4, M7, and M9) across all three binding sites of the CRD (3LL2) defined by residues (11–12 and 108–110), (89–90 and 66–68), and (44 and 26–28) (Fig. 6). The third binding site mannose was donated from a symmetry related molecule in the crystal [19].
Figure 6. GRFT WT and Monomeric Engineered mGRFT.
A) WT GRFT is rendered in cartoon with the domain-swapped β-strands highlighted in orange and olive. B) Engineered monomeric mGRFT represented as cartoon highlighting the lack of domain-swap with the CRD colored red. PDBID 3LL2 was used to show the CRD. The GS insertion is represented as black spheres with the unswapped portion in orange. C) Close up of the CRD with the residues that make up the active site in red and the glycan in stick representation.
Griffithsin was initially identified in an anti-HIV-1 screen with EC50 of 0.43–0.98 nM for HIV-1 laboratory strain and primary isolates [79]. GRFT was also tested against numerous other viruses, including other HIV-1 subtypes (0.02–160 nM), HIV-2 (2–33 nM), HCV (0.6–7.6 nM), HSV-2 (0.181–11.26 μM), SARs-CoV (0.048–960 nM), various avian CoV subtypes (0.032–0.57 nM), Bovine coronavirus (0.057 nM), avian infectious bronchitis virus (0.032 nM) mouse hepatitis virus (0.23 nM) puffinosis coronavirus (0.57 nM), human coronavirus (HCoV) and mutants (0.16 nM), Japanese encephalitis virus (JEV) (20 nM) Simian immunodeficiency virus(0.95–1.24 nM), chimeric Simian/human immunodeficiency virus (0.02–0.83 nM), and Ebola virus (results soon to be published) with the greatest potencies against HIV-1 and 2 and HCoV (NL63) with a range of IC50 of 0.03–181 nM [36, 57, 79, 81–87].
a. In vivo Parenteral Therapeutic
The lectin was tested in a number of in vivo models against HIV-1 and HCV. GRFT was systemically distributed throughout sera and plasma after subcutaneous injection with anti-HIV-1 activity preserved in sera when tested in a follow-up cell-based antiviral assay [88]. Minimal toxicity was observed by a range of single and repeat s.c. daily doses of GRFT in two rodent species and rGRFT (plant recombinant) was not inflammatory or irritating in the rabbit vaginal irritation model, supporting the therapeutic potential of the lectin [89]. Subcutaneously administered GRFT was also efficacious against HCV in mouse-human chimeric liver models and was readily bioavailable in plasma at 3.3 mg/mL on day 11 and slow reduction to 0.59 mg/mL on day 18 [41]. GRFT was well tolerated by systemically treated mice at daily doses of 5 and 20 mg/kg. Finally, intraperitoneally administered GRFT (5 mg/kg) prior to viral presentation completely inhibited JEV infection/mortality in mouse models [82].
b. In vivo Preventative Microbicide
GRFT also blocked cell-to-cell transmission of HSV-2, protecting mice from genital herpes through a single dose intravaginal application of a 0.1% GRFT gel [81]. The GRFT gel was not cytotoxic and did not prevent the mucosal response. Intranasal administration of GRFT at 10 mg/kg protected against SARS-CoV in a lethal mouse model of pulmonary infection and prevented weight loss, improved lung histopathology, and reduced lung tissue virus titers [80, 86]. According to NIH RePORT, clinical trials are anticipated for 2017 with the active agent GRFT formulated as a rectal microbicide gel to prevent viral entry of HIV types 1 and 2, as well as HSV-2 and HCV (Project Number: 5U19AI113182-03) [90].
c. Engineering Efforts
Not only has GRFT exhibited potent virucidal activities against a range of enveloped viruses, the lectin is also stable at elevated temperatures and a broad range of pH and is amenable to large-scale preparation in recombinant systems [89]. Research efforts to generate monomeric forms of GRFT (mGRFT) resulted in decrease in antiviral potency (up to 323 nM) suggesting the oligomeric state and distance between CRDs strongly influence virucidal activity (Fig. 6) [19]. The monomeric form of the lectin does not participate in the domain swap of the first two β-strands and was engineered through the insertion of Gly-Ser at position 17 localized to the random coil between strand 1 and 2 (Fig. 6).
Engineering of tandemeric mGRFT with 2, 3, and 4 mGRFT linked with variable length linkers resulted in 3mGRFT and 4mGRFT with anti-HIV activities with an EC50 of 1 and 1.2 pM, respectively [91]. Examination of anti-HIV activities against various subtype A, B, and C strains with the mGRFT tandemers revealed better global anti-HIV activities with median IC50 values of 0.396, 0.298, and 0.181 nM, respectively as compared to both mGRFT and wtGRFT. Isothermal Titration Calorimetry binding studies revealed the 2mGRFT3 and 3mGRFT tandemers bind glycosylated gp120 (from HIV-1) with 3 nM affinity, which was slightly better than GRFT and almost 2 orders of magnitude better than mGRFT alone. The tandemeric 3mGRFT represents the most potent antiviral lectin discussed herein with in vivo characterization that makes it an outstanding candidate for clinical use as topical microbicide and s.c. therapeutic.
B. Monocot consensus fold - β-Prism Type-II
Lectins that adopt the monocot consensus fold contain three 4-stranded anti-parallel β-sheets arranged with approximate 3-fold symmetry resulting in a β-prism type II fold with 3 CRDs per monomer [92]. β-prism II fold lectins contain a conserved hydrophobic core that is paramount for structure and a conserved mannose binding sequence (QXDXNXVXY) [93]. The monocot consensus fold lectins discussed in this review have specificity for α(1,3),α(1,6) linked mannoses (Table 4). Members of this class of lectin are biosynthesized as a 160 residue preprotein that is post-translationally cleaved at the N and C termini resulting in the mature 110 residue pro-protein and participate in C-terminal strand exchange.
1. Scilla campanulata Lectin (SCL)
Scilla campanulata
Lectin (SCL) was isolated from the bulbs of the Spanish blue-bell and has a monomeric molecular weight of 13.1 kDa containing 119 residues that exists as a tetramer in solution [94]. The lectin was reported to have moderate anti-HIV activity with EC50 values of 4.6 and 8 μM for HIV-1 and HIV-2, respectively [95]. The protein adopts a monocot consensus fold in published crystal structures and was reported as having mannose specificity with higher preference for α(1,3),α(1,6)-mannotriose as determined through inhibition studies (250 μM) [95]. Although the crystal structure did not contain any mannose, alignment of SCL, NPL, and GNA primary sequences revealed the conserved monocot consensus CRD signature.
2. Narcissus pseudonarcissus Lectin (NPL)
Narcissus pseudonarcissus
Lectin (NPL), also known as Narcissus pseudonarcissus Agglutinin, was isolated from daffodil bulbs [96]. The lectin consists of 109 residues with a monomeric molecular weight of 12 kDa and in solution was reported to form dimeric, trimeric, and tetrameric oligomers with crystallographic and SAXS structures, while size-exclusion experiments supported the tetrameric assembly [97]. NPL monomers have 3 CRD (and a fourth, low affinity CRD) and adopt the monocot consensus fold with residues 102–109 participating in strand exchange [same ref as above]. Mannose binds the CRD of NPL through interaction with the side chains of Q26, D28, N30, and Y34, which are conserved across the three CRDs. The lectin is reported to have anti-HIV-1 and 2 and anti-SIV activities with EC50s 0.7–7.3 μg/ml (~50–500 nM) and, to a lesser extent, anti-HSV, anti-Rabies, and anti-Rubella activity with MIC50s of 11, 8.2, and 2.9 μM, respectively [98–100]. Literature supports that the antiviral activities derive from the specific interaction with α1,3 or α1,6 linked mannoses which is consistent with this lectin family [98–100].
3. Galanthus nivalis Agglutinin (GNA)
Galanthus nivalis
Agglutinin (GNA) was isolated from the bulbs of snowdrop (Galanthus nivalis) [101]. The lectin has a monomeric molecular weight of 12.5 kDa and adopts a tetrameric state in solution [102]. Crystal structures revealed that the protein adopts the monocot consensus fold with conserved CRD residues (1NIV: Q89, D91, N93, and Y97) when compared to oligosaccharide bound NPL structure [103, 104]. The lectin is reported to have anti HIV-1 and 2, SIV, feline immunodeficiency virus (FIV), non-nucleoside reverse transcriptase inhibitor (NNRTI)-resistant HIV-1, Lamivudine-, Zidouvudine-, and PI-resistant HIV-1 with a range of EC50 values of 0.09–4.7 μg/ml [99, 105]. Solution binding studies revealed that the lectin prefers oligosaccharides containing α1,3 or α1,6 linked mannoses with some decreased affinity for amylose, dextran, and glycogen [101]. Like the antiviral lectins previously discussed, the antiviral activities are thought to derive specifically from the interaction with the mannose of glycoprotein gp120. Furthermore, GNA did not illicit a mitogenic response in PBMC cultures (100 μg/mL) and intravenously administered GNA in NMRI mice at 100 mg/kg bolus injection resulted in no measurable toxic effects [105].
4. Hippeastrum Hybrid Agglutinin (HHA)
Hippeastrum
Hybrid Agglutinin (HHA) was isolated from the bulbs of amaryllis (Hippeastrum hybrid) [96]. The protein is ~12.5 kDa and exists as a tetramer in solution. The lectin is reported to have anti-HIV-1 and 2, SIV, FIV, NNRTI-resistant HIV-1, Lamivudine-, Zidouvudine-, and PI-resistant HIV-1 with a range of EC50 values of 0.16–3.2 μg/ml [105]. Surface Plasmon Resonance studies confirmed that the lectin interacts specifically with the gp120 viral envelope protein with a dissociation constant of 0.39 and 0.55 nM for monomeric WT gp120 and trimeric gp140, respectively [106]. Although no PDBID exists for this particular protein, the crystal structure of HHA has been reported [107]. The structure was solved through molecular replacement using the coordinates from the monocot consensus fold adopting lectin GNA supporting that HHA adopts the monocot consensus fold. Similar to GNA, HHA did not illicit a mitogenic response in PBMC cultures (100 μg/mL) and intravenously administered GNA in NMRI mice at 100 mg/kg bolus injection resulted in no measurable toxic effects [105].
5. Polygonatum cyrtonema Lectin (PCL)
Polygonatum cyrtonema lectin (PCL) was isolated from the rhizomes of the traditional Chinese medicinal herb, P. cyrtonema Hau [108]. The 12 kDa protein contains 110 residues, exists as a dimer in solution and X-ray crystal structures resolve bound α(1,3)-dimannoside (3A0E) interacting with the monocot CRD consensus sequence, which supports that the lectin anti-HIV activity stems from an interaction with the glycans of gp120 and/or gp41 [109]. PCL was reported to have anti-HIV-1 and 2 activities with EC50 values of 0.05–0.10 μg/ml making PCL the most potent monocot consensus fold lectin discussed in this review [108].
C. Concanavalin (ConA) – 13-stranded anti-parallel β-sheet Ca2+ and Mn2+ binding
Concanavalin A (ConA) was originally isolated from the jackbean Canavalia ensiformis [110]. ConA belongs to the legume lectin family. The monomeric weight of the lectin is 25 kDa and exists as a tetramer in solution [111]. More than 50 structures are present in the PDB each revealing the similar fold of 13 anti-parallel β-strands adopting 2 β-sheets comprised of 6 and 7 β-strands, which also bind Ca2+ and Mn2+ [112]. Interestingly, only one CRD is defined per monomer with a total of 4 CRDs present in the intact tetramer. Mannose binds the CRD through interactions with D14, L99, Y100, R228 amide hydrogen bonding as well the carboxylate side chain of D208 (O1-Methyl-mannose; 5CNA) [113].
ConA was reported to have anti-HIV-1 activity with EC50 of 2.2–2.6 μg/mL and anti-HSV activity with an IC50 of 98 nM [35, 114]. ConA binding requires GlcNAc, terminal α-mannose residues, as well as branched mannose (G1, G2, M1, M5, M8) suggesting a similar, but more inclusive, interaction as MVL [115]. ConA was reported to interact non-specifically with various glycoforms of gp120 suggesting that the lectin is less sensitive to mutations in glycosylation locations in the envelope protein than the monoclonal antibody 2G12 [116].
D. Hevein-like
1. Urtica dioica Agglutinin (UDA)
Urtica dioica
Agglutinin (UDA) was isolated from stinging nettle (Urtica dioica) and is monomeric with a molecular weight of 8.5 kDa [117, 118]. The lectin has two internal repeats with 46% sequence identity with R1 including residues 1–46 and R2 including residues 47–90. UDA adopts 2 hevein-like domains containing 4 disulfide bonds per domain with 1 CRD per domain. The crystal structure 1EN2 with bound tetrasaccharide GlcNac revealed that the carbonyl of C24 hydrogen bonds to a hydroxyl on GlcNac1 with some hydrophobic interactions between the same sugar and W23, the hydroxyl of S19 hydrogen bonds to the acetate of GlcNac2, the carbonyl of Y30 hydrogen bonds to GlcNac 2, hydrophobic interactions between W21 and GlcNac3, and hydrogen bonding between W21 and hydroxyl on GlcNac4 [119, 120]. UDA CRD has specific interactions for each of the 4 sugars used in the co-crystallization experiment and represents a core glycan binding specificity that is not seen with the other lectins mentioned in this review (G1 G2).
Antiviral activity has been reported for HIV-1 and 2, cytomegalovirus (CMV), respiratory syncytial virus (RSV), Influenza A, and SARS-CoV with a range of IC50 values of ~100 nM – 1 μM [121, 122]. UDA specifically interacts with oligomers of GlcNAc presumably at the core of glycans on gp120/41 and homologous envelope proteins.
a. In vivo Parenteral Therapeutic
UDA was tested against SARS-CoV infected BALB/c mice demonstrating that 5 mg/kg/day significantly protected the mice against lethal infection, reduced lung pathology scores, and protected against weight loss, but did not reduce viral lung titers [123]. Therefore, UDA represents a moderately active antiviral lectin with increased therapeutic utility due to the lack of mitogenic response. Perhaps efforts at increasing specificity or testing against other enveloped viruses can increase the utility of this lectin.
2. Myrianthus holstii Lectin (MHL)
Myrianthus holstii
Lectin (MHL) was isolated from the roots of the African plant Myrianthus holstii [124]. The 9.2 kDa lectin has anti-HIV-1 activity (HIV-1-RF) in infected CEM-SS cells with an EC50 of 150 nM and exhibited GlcNAc specificity. Although no structure is currently available, the protein is thought to adopt a fold similar to UDA due to the strong 61% sequence identity and similar glycan specificity.
E. Nicotiana tabacum Agglutinin (NICTABA) – Structure Unknown
NICTABA was first identified in the leaves of Nicotiana tabacum var. Samsun NN in response to treatment with jasmonates or insect infestations [125]. The 19 kDa lectin adopts a dimer in solution (38 kDa) and has reported antiviral activities against HIV-1/2 (5–30 nM), HSV (53–263 nM), Influenza A/B (11–32 nM), and RSV (105 nM) and was found to be inactive against non-enveloped viruses [126]. NICTABA specifically interacts with GlcNAc oligomers and high-mannose type glycans and surface plasmon resonance with HIV-1 gp41 and gp120 revealed kD of 1.5 × 10−9 and 3.8 × 10−9 M, respectively. NICTABA did not affect the binding between mAb 2G12 and HIV-1 suggesting an alternate interaction with gp120 as compared to HHA and UDA. The lectin inhibits syncytium formation and DC-SIGN directed transmission CD4+ T-lymphocytes. The median cytotoxic concentration of NICTABA is greater than 100 μg/ml against various cell-lines. Despite the abundance of putative homologous sequences in many flowering plant genomes, no structural information currently exists. NICTABA is considered the prototype for a ubiquitous family of lectins belonging to plants [125].
V. Antiviral lectins obtained from marine eukaryotic organisms
The marine derived eukaryotic antiviral lectins have little structural information available. Specifically, CGL derived from the marine mussel Crenomytilus grayanus is the only marine lectin with a published structure (β-trefoil). The antiviral activities, structural classes, and glycan specificities are summarized in Tables 5 and 6.
Table 5.
Reported Antiviral Activities of Marine Derived Antiviral Lectins.
| Lectin | Source | Activity | Cytotoxicity | References |
|---|---|---|---|---|
| CVL | Chaetopterus variopedatus | HIV-1 (4.3 nM) | No | [127, 128] |
| SVL-1 | Serpula vermicularis | HIV-1 (89.1 μg/mL) | Unk | [129, 130] |
| SVL-2 | Serpula vermicularis | HIV-1 (0.15 μg/mL) | Unk | [129, 130] |
| CGL | Crenomytilus grayanus | HIV-1 (27.8 μg/mL) | Yes | [130, 131, 133] |
| DTL | Didemnum ternatanum | HIV-1 (0.2 nM) | No | [130, 137] |
| DTL-A | Didemnum ternatanum | HIV-1 (42 nM) | No | [130, 137] |
| GSL | Savaglia savaglia | HIV-1 (200 nM) | No | [138, 139] |
A. Lectin from marine worm Chaetopterus variopedatus (CVL) – Structure Unknown
CVL was isolated from the marine worm Chaetopterus variopedatus [127]. The 30 kDa protein was reported to inhibit HIV-1 syncytium formation with an EC50 of 4.3 nM and did not elicit any cytopathic effects in C8166 cells at the concentrations tested (0.003–1.67 μM) [128]. Carbohydrate specificity of CVL was assessed through the inhibition of CVL mediated hemagglutination with monosaccharide, oligosaccharide, and glycoprotein and resulted in no hemagglutinin inhibition with mannose monosaccharides, but rather saccharides containing galactose.
B. Lectins from Serpula vermicularis – Structure Unknown
Serpula vermicularis
Lectin 1 and 2 (SVL-1/2) were isolated from the sea worm Serpula vermicularis [129, 130]. SVL-1 is ~65 kDa homotetrameric protein with a carbohydrate preference for mannose. The protein was reported as having anti-HIV-1 activity with an EC50 of 89.1 μg/mL. SVL-2 is ~50 kDa homotetrameric protein with a monomeric weight of 12.7 kD. Carbohydrate content was calculated by the phenol-sulfuric acid method revealing that 1.9% of the molecular weight derives from carbohydrate, suggesting that the lectin is a glycoprotein. SVL-2 has been shown to prefer GlcNAc as indicated from the inhibition of SVL-2 hemagglutination by various saccharides. SVL-2 blocked the upregulation of viral p24 antigen and inhibited HIV-1 infectivity with EC50s of 0.23 and 0.15 μg/ml, respectively.
C. Lectin from marine mussel – β-trefoil
Crenomytilus grayanus lectin (CGL) was isolated from the sea mussel, Crenomytilus grayanus, collected from the Great Bay of the Sea of Japan [131]. The protein contains 150 residues with a molecular weight of 18 kDa with three internal repeats with ~73% similarity. Crystal structures revealed that CGL adopts the β-trefoil type fold with the three CRDs having a conserved sequence of H,Y/K, GGVHDH, R/A [132, 133]. The protein is dimeric in crystallo and size-exclusion chromatography. CGL was shown to interact with mucin-type receptors and reported to have carbohydrate preference for GalNAc/Gal [131, 134]. NMR titration experiments resulted in the calculated KD for galactose and galactosamine for CRDs 1, 2, and 3 of 32–178, 815–5135, 302–1288 μM, respectively, with CRD 1and 3 preferring galactosamine, and CRD2 preferring galactose [133]. Modest anti-HIV-1IIIB activity was reported with an EC50 of 27.88 μg/mL [130]. Bacterial agglutination and fungal hyphal growth suppression were also reported for CGL that were inhibited with increasing concentrations of galactose [135, 136]. Finally, CGL was reported as having low anticancer activity against breast cancer (MCF7) in a MTT assay >200 μg/mL [133]. CGL might be generally cytotoxic due to the mild activity against a myriad of organisms.
D. Lectins from Ascidians – Structures Unknown
Didemnum ternatanum
Lectin (DTL) and Didemnum ternatanum Lectin-A (DTL-A) were isolated from the ascidian Didemnum ternatanum [137]. DTL was reported to as being homotrimeric with a molecular weight of 27 kDa and reduced molecular weight of 10 kDa. Hemmaglutination activity was inhibited by addition of GlcNAc, chitobiose, and desialylated glycoproteins [137]. DLT-A has a molecular weight of 14 kDa and is capable of forming high-molecular aggregates. The lectin was reported to interact with GlcNAc and GalNAc and it was suggested to have two independent binding sites with altered specificities for the different sugars [130]. DTL and DTL-A were reported to have anti-HIV-1 IIIB activity with EC50 of 0.2 and 42 nM, respectively. DTL inhibited 50% of p24 antigen synthesis at 0.006 μg/mL and exhibited no cytotoxicity (C8166 cells) >500 μg/mL. DTL-A was less effective against HIV than DTL by approximately 2 orders of magnitude.
E. Coral-derived – Structure Unknown
The Gerardia savaglia Lectin (GSL) was isolated from the marine coral Savaglia savaglia and is reported to be heterodimeric with a molecular weight of 14.8 kDa [138]. The 121 residue protein is rich in acidic amino acids with a pI of 4.8 and does not contain any cysteines as identified from amino acid analysis. The lectin inhibited nuclear mRNA translocation in vitro and was reported to have anti-HIV-1 activity of 200 nM with no mitogenicity at 50× that concentration [139]. GSL inhibited syncytia formation in an HIV-1 human lymphocyte system and was shown to interact with the glycans of gp120. Loss of anti-HIV-1 activity was observed in the presence of 500:1 D-mannose, which further supports GSL interaction with high-mannose glycans on gp120. No primary sequence or structural information is available.
VI. Considerations in the clinical development of antiviral lectins
The challenge for the clinical use of heterologous lectins in human antiviral prophylaxis and therapy derive, not from difficulties in inhibiting viral entry, but from the pharmacological hurdles of administering a foreign protein into the human system. Whether or not any of the lectins described above will eventually be useful clinically will depend on a number of physiological factors we discuss below. At this point in their development, antiviral lectins are only being pursued clinically as anti-STD (primarily HIV) microbicides via mucosal administration. Additional systemic efficacy for acute infections has been demonstrated for subcutaneous administration but much less pre-clinical data has been generated about the use of antiviral lectins in this manner. Some of the factors described below relate specifically to systemic use (e.g. bioavailability) while others are important for all administration routes (e.g. toxicity/immunogenicity and affordability).
A. Bioavailability
The first hurdle for the clinical utility of antiviral lectins is assessment for their bioavailability following administration. As proteins, it is assumed that lectins will not be able to be orally administered as they would not survive digestive enzymes. Few studies have assessed the systemic bioavailability of lectins. Two lectins that have been assessed are CV-N and GRFT. CV-N was found to be biologically available following subcutaneous injection into mice [37] with dosing up to 5.6 mg/kg/day tolerated. The bioavailability of CV-N was assessed in the context of studies evaluating its systemic efficacy against Ebola Zaire (EBOZ) where CV-N was shown to delay the mean time to death for treated animals by several days. As the animals were challenged by intraperitoneal injection with EBOZ, the combined results indicated that CV-N was bioavailable and retained activity following subcutaneous injection.
There is a larger sample of pertinent data on the bioavailability of GRFT. Initial studies on GRFT concentrated on its stability in biological fluids. GRFT was shown to be stable in either buffered solutions at pH 4–8 or in cervical vaginal lavage fluid for up to 1 week at either 25°C or 37°C with no loss of activity [87]. Further evaluation of the physiological stability of GRFT revealed that GRFT was resistant to degradation by 8/9 human proteases in the experiment and showed the greatest resistance to protease degradation of the antimicrobial peptides tested [140]. Only the elastase family of human proteases was found to moderately degrade GRFT.
A study that directly measured the bioavailability and distribution of GRFT following subcutaneous injection in both mice and guinea pigs was also reported [88]. It was shown that GRFT is fully biologically available in mice following a single subcutaneous injection of 50 mg/kg with plasma levels reaching up to 4 nM on day 1, reducing to 0.5 nM by day 7. A second study in which mice were injected subcutaneously with 10 mg/kg each day for 10 days showed a steady increase in GRFT plasma levels reaching a peak of 25 nM on day 11 and persisting at levels of >4 nM one week after cessation of administration. GRFT concentrations in the sera of guinea pigs after similar 10 mg/kg/day treatments reached levels of 36 nM on day 11 reducing to ~11 nM on day 15, 5 days after cessation of treatment. This study also found that GRFT distributed into tissues with kidney, liver and spleen tissues showing levels of approximately 4, 2 and 6 μg/g of tissue respectively. It should be noted that plasma samples taken from test animals showed that GRFT retained anti-HIV activity in plasma following subcutaneous administration out to day 15.
Additional reports of the efficacy of both GRFT and SVN include activity against hepatitis C following subcutaneous injection [41, 83] and, for GRFT against Japanese encephalitis virus following intraperitoneal injection [82]. SVN was also reported to be bioavailable (plasma levels up to 1 μg/ml) and display in vivo efficacy against EBOZ following subcutaneous injection into mice [58]. These reports provide further evidence that antiviral lectins can be both bioavailable and active in vivo.
B. Toxicity and immunogenicity
A major concern for any clinical agent is the chance for toxicities unrelated to the target of the active agent. Though antiviral lectins are targeted at oligosaccharides present on viral envelope glycoproteins, these oligosaccharides are synthesized and attached by host cell enzymes, which means that they can also be present on human cells. Potential side effects from the systemic use of lectins could arise from the well-established ability of lectins to agglutinate cells [141]. Therefore, it is incumbent upon researchers to test antiviral lectins for their ability to agglutinate haemopoeitic cells prior to proposing systemic use.
Other toxicities could arise from acute immunologic response to the administration of a foreign protein (anaphylaxis) [142]. Such immunogenicity is perhaps the greatest concern in the clinical use of foreign proteins in humans. A handful of current examples of heterologous proteins used in human clinical treatment exist including the cone snail peptide Ziconotide for chronic pain [143], the Gila monster saliva peptide Exenatide, systemically administered for Type II diabetes [144] and, most prominently, the microbial product botulinum toxin [145]. All of these proteins have been used successfully in the clinic for years with acceptable risk profiles, giving encouragement to those who endeavor to discover additional biologically active proteins and peptides.
Of the antiviral lectins discussed in this review, many have been assessed in vivo for either toxicity or immunogenicity. Concanavalin A was identified clearly as a causative agent in acute liver injury in mice [146] due to its binding to sinusoidal endothelial cell which then causes these cells to be attacked by CD4+ T-cells [147] ultimately leading to a cascade of damage to the liver and death in mice. Other antiviral lectins reported to have unacceptable toxicities include UDA, WGA, NPL and Jacalin. The clear potential for such adverse effects has necessitated that all new antiviral lectins be tested for both agglutination and immunogenicity.
A final component of the potential adverse effects of antiviral lectins relates directly to their potential use as anti-HIV agents and their status as foreign proteins in the human system. Heterologous proteins have the potential to be stimulatory to the immune system. This is often demonstrated by their ability to activate T-cells or PBMCs. Such immune cell activation can stimulate increased amounts of CD4+ HIV target cells near sites of administration (both topical and systemic). The increased level of target cells can have the adverse effect of actually increasing the likelihood of HIV infection. Concern over this adverse effect has been raised for several lectins including ConA and CV-N [34].
C. Routes of administration
Antiviral lectins, due to their proteinaceous nature, have not been reported to be biologically-available following oral administration. This has necessitated their administration by alternate routes. Reports of antiviral efficacy for antiviral proteins have included those administered topically [89] [46], those administered systemically either by subcutaneous [37, 88] or intraperitoneal [148] injections, and by intranasal administration to prevent respiratory infections [86, 149]. Additional administration methods have included such disparate mechanisms as the vaginal delivery of genetically-engineered lactobacilli secreting CV-N through both direct suppository administration (vaginally) [150] and the feeding of yogurt (to provide rectal administration) [151]. The variety of administration methods indicates the robust efforts so far expended to insure that potent antiviral lectins can reach their sites of action intact.
D. Affordability
A final concern for the clinical utility of antiviral proteins is their cost. Most of the viruses for which these lectins have been put forward as potential clinical agents are either emerging infectious agents such as Ebola, SARS CoV, and Japanese encephalitis or as microbicidal agents acting against sexually transmitted viruses such as HIV and HSV. The economic realities of these particular utilities require that these antiviral proteins be produced at a reasonable cost. When one considers that the societies most impacted by the current HIV epidemic are predominantly those with minimal health resources for their patient population the need for low cost production strategies is clear. To address this need, proteins such as GRFT and CV-N have been produced in genetically engineered plants including tobacco [89, 152], soy [153] and rice [154, 155]. The cost advantages of large-scale plant-based production offer an opportunity to effectively bring antiviral proteins to the clinic [156].
VII. Potential for further improvements
As with many clinically used small molecule natural products, antiviral proteins have the potential to be improved by changes to their structure that will render them more suitable for clinical development. The structural information now available for these proteins has enabled rational design of mutant proteins with improved capabilities. In some cases this has been the design of more potent analogs as demonstrated by GRFT tandemers, which were engineered to allow more conformational freedom between CRDs and resulted in a 5–10 fold improvement in anti-HIV activity and enhanced activity against GRFT-resistant strains of HIV [91]. These GRFT tandemers were able to show similar activity against HIV strains lacking oligosaccharides at positions N234 and N295 on the HIV-1 subtype C Env glycoproteins which renders the virus resistant to the monomeric and WT GRFT forms (Fig. 1) [36].
Other modifications affect the immunogenicity of antiviral proteins including the modification of CV-N with polyethylene glycol (PEG) to reduce immunogenicity and increase its half-life in serum [157] and the genetic engineering of BanLec to reduce the presence of antigenic epitopes [75, 76] and reduce its immunogenicity. The H84T mutation in BanLec is thought to mediate mitogenicity by imparting decreased affinity for complex sugars. Hybrid lectins have also been produced from PFA and OAA to render a protein OPA with improved activity [63]. Other modifications in structure have produced obligate monomeric [19] or dimeric [158] proteins in an effort to improve clinical potential. Significant research into these potentially useful modifications is necessary to truly develop these agents for human use.
Highlights.
Many natural lectins have been reported to have antiviral activity.
This article reviews source organisms for antiviral lectins.
We discuss structural classes and carbohydrate specificity for various antiviral lectins.
We detail the antiviral activity reported for various natural lectins.
We discusse challenges to clinical utility for heterologous proteins.
Acknowledgments
This work was supported by funds from the NIH Intramural AIDS Targeted Antiviral Program, the Center for Cancer Research at the National Cancer Institute and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boerwinkel DF, et al. Fluorescence imaging for the detection of early neoplasia in Barrett’s esophagus: old looks or new vision? Eur J Gastroenterol Hepatol. 2014;26(7):691–8. doi: 10.1097/MEG.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 2.Belicky S, Katrlik J, Tkac J. Glycan and lectin biosensors. Essays Biochem. 2016;60(1):37–47. doi: 10.1042/EBC20150005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimitrov DS. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol. 2004;2(2):109–22. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzarini J, et al. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J Virol. 2004;78(19):10617–27. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolchinsky P, Kiprilov E, Sodroski J. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J Virol. 2001;75(5):2041–50. doi: 10.1128/JVI.75.5.2041-2050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Keefe BR, et al. Analysis of the interaction between the HIV-inactivating protein cyanovirin-N and soluble forms of the envelope glycoproteins gp120 and gp41. Mol Pharmacol. 2000;58(5):982–92. doi: 10.1124/mol.58.5.982. [DOI] [PubMed] [Google Scholar]
- 7.Botos I, Wlodawer A. Cyanovirin-N: a sugar-binding antiviral protein with a new twist. Cell Mol Life Sci. 2003;60(2):277–87. doi: 10.1007/s000180300023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokesch HR, et al. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42(9):2578–84. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- 9.Pancera M, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514(7523):455–61. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchdoerfer RN, et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531(7592):118–21. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, et al. Toremifene interacts with and destabilizes the Ebola virus glycoprotein. Nature. 2016;535(7610):169–72. doi: 10.1038/nature18615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinter A, et al. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63(6):2674–9. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earl PL, Doms RW, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1990;87(2):648–52. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart-Jones GB, et al. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell. 2016;165(4):813–26. doi: 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones PL, Korte T, Blumenthal R. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J Biol Chem. 1998;273(1):404–9. doi: 10.1074/jbc.273.1.404. [DOI] [PubMed] [Google Scholar]
- 16.Davenport YW, West AP, Jr, Bjorkman PJ. Structure of an HIV-2 gp120 in Complex with CD4. J Virol. 2015;90(4):2112–8. doi: 10.1128/JVI.02678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrientos LG, et al. Dissecting carbohydrate-Cyanovirin-N binding by structure-guided mutagenesis: functional implications for viral entry inhibition. Protein Eng Des Sel. 2006;19(12):525–35. doi: 10.1093/protein/gzl040. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, et al. Multivalent interactions with gp120 are required for the anti-HIV activity of Cyanovirin. Biopolymers. 2009;92(3):194–200. doi: 10.1002/bip.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulaei T, et al. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure. 2010;18(9):1104–15. doi: 10.1016/j.str.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi A, et al. Actinohivin: specific amino acid residues essential for anti-HIV activity. J Antibiot (Tokyo) 2010;63(11):661–5. doi: 10.1038/ja.2010.106. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, et al. Mechanism by which the lectin actinohivin blocks HIV infection of target cells. Proc Natl Acad Sci U S A. 2009;106(37):15633–8. doi: 10.1073/pnas.0907572106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bewley CA, et al. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat Struct Biol. 1998;5(7):571–8. doi: 10.1038/828. [DOI] [PubMed] [Google Scholar]
- 23.Shahzad-ul-Hussan S, et al. Solution structure of the monovalent lectin microvirin in complex with Man(alpha)(1–2)Man provides a basis for anti-HIV activity with low toxicity. J Biol Chem. 2011;286(23):20788–96. doi: 10.1074/jbc.M111.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams DC, Jr, et al. Crystal structures of the HIV-1 inhibitory cyanobacterial protein MVL free and bound to Man3GlcNAc2: structural basis for specificity and high-affinity binding to the core pentasaccharide from n-linked oligomannoside. J Biol Chem. 2005;280(32):29269–76. doi: 10.1074/jbc.M504642200. [DOI] [PubMed] [Google Scholar]
- 25.Moulaei T, et al. Atomic-resolution crystal structure of the antiviral lectin scytovirin. Protein Sci. 2007;16(12):2756–60. doi: 10.1110/ps.073157507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiba H, et al. Actinohivin, a novel anti-HIV protein from an actinomycete that inhibits syncytium formation: isolation, characterization, and biological activities. Biochem Biophys Res Commun. 2001;282(2):595–601. doi: 10.1006/bbrc.2001.4495. [DOI] [PubMed] [Google Scholar]
- 27.Chiba H, et al. Actinohivin, a novel anti-human immunodeficiency virus protein from an actinomycete, inhibits viral entry to cells by binding high-mannose type sugar chains of gp120. Biochem Biophys Res Commun. 2004;316(1):203–10. doi: 10.1016/j.bbrc.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Zhang F, et al. The characteristic structure of anti-HIV actinohivin in complex with three HMTG D1 chains of HIV-gp120. Chembiochem. 2014;15(18):2766–73. doi: 10.1002/cbic.201402352. [DOI] [PubMed] [Google Scholar]
- 29.Hoorelbeke B, et al. Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specifically targeting high-mannose-type glycans on the gp120 envelope. Antimicrob Agents Chemother. 2010;54(8):3287–301. doi: 10.1128/AAC.00254-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matoba N, et al. HIV-1 neutralization profile and plant-based recombinant expression of actinohivin, an Env glycan-specific lectin devoid of T-cell mitogenic activity. PLoS One. 2010;5(6):e11143. doi: 10.1371/journal.pone.0011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd MR, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41(7):1521–30. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustafson KR, et al. Isolation, primary sequence determination, and disulfide bond structure of cyanovirin-N, an anti-HIV (human immunodeficiency virus) protein from the cyanobacterium Nostoc ellipsosporum. Biochem Biophys Res Commun. 1997;238(1):223–8. doi: 10.1006/bbrc.1997.7203. [DOI] [PubMed] [Google Scholar]
- 33.Yang F, et al. Crystal structure of cyanovirin-N, a potent HIV-inactivating protein, shows unexpected domain swapping. J Mol Biol. 1999;288(3):403–12. doi: 10.1006/jmbi.1999.2693. [DOI] [PubMed] [Google Scholar]
- 34.Balzarini J, et al. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J Virol. 2006;80(17):8411–21. doi: 10.1128/JVI.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witvrouw M, et al. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol. 2005;79(12):7777–84. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexandre KB, et al. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin. Virology. 2010;402(1):187–96. doi: 10.1016/j.virol.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrientos LG, et al. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antiviral Res. 2003;58(1):47–56. doi: 10.1016/s0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 38.O’Keefe BR, et al. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47(8):2518–25. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kachko A, et al. Inhibition of hepatitis C virus by the cyanobacterial protein Microcystis viridis lectin: mechanistic differences between the high-mannose specific lectins MVL, CV-N, and GNA. Mol Pharm. 2013;10(12):4590–602. doi: 10.1021/mp400399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helle F, et al. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J Biol Chem. 2006;281(35):25177–83. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 41.Takebe Y, et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS One. 2013;8(5):e64449. doi: 10.1371/journal.pone.0064449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolmstedt AJ, et al. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol Pharmacol. 2001;59(5):949–54. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- 43.Shenoy SR, et al. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J Pharmacol Exp Ther. 2001;297(2):704–10. [PubMed] [Google Scholar]
- 44.Bewley CA, Otero-Quintero S. The potent anti-HIV protein cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to Man(8) D1D3 and Man(9) with nanomolar affinity: implications for binding to the HIV envelope protein gp120. J Am Chem Soc. 2001;123(17):3892–902. doi: 10.1021/ja004040e. [DOI] [PubMed] [Google Scholar]
- 45.Tsai CC, et al. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20(1):11–8. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 46.Tsai CC, et al. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003;19(7):535–41. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- 47.Cohen CR. Preclinical Development of Mucocept. 2016 Available from: https://projectreporter.nih.gov/project_info_description.cfm?aid=9121468&icde=; Project Number: 5U01AI123082-02.
- 48.Kehr JC, et al. A mannan binding lectin is involved in cell-cell attachment in a toxic strain of Microcystis aeruginosa. Mol Microbiol. 2006;59(3):893–906. doi: 10.1111/j.1365-2958.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- 49.Bewley CA. Solution structure of a cyanovirin-N:Man alpha 1–2Man alpha complex: structural basis for high-affinity carbohydrate-mediated binding to gp120. Structure. 2001;9(10):931–40. doi: 10.1016/s0969-2126(01)00653-0. [DOI] [PubMed] [Google Scholar]
- 50.Huskens D, et al. Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J Biol Chem. 2010;285(32):24845–54. doi: 10.1074/jbc.M110.128546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi M, et al. Isolation and characterization of a mannan-binding lectin from the freshwater cyanobacterium (blue-green algae) Microcystis viridis. Biochem Biophys Res Commun. 1999;265(3):703–8. doi: 10.1006/bbrc.1999.1749. [DOI] [PubMed] [Google Scholar]
- 52.Bewley CA, et al. New carbohydrate specificity and HIV-1 fusion blocking activity of the cyanobacterial protein MVL: NMR, ITC and sedimentation equilibrium studies. J Mol Biol. 2004;339(4):901–14. doi: 10.1016/j.jmb.2004.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shahzad-ul-Hussan S, Cai M, Bewley CA. Unprecedented glycosidase activity at a lectin carbohydrate-binding site exemplified by the cyanobacterial lectin MVL. J Am Chem Soc. 2009;131(45):16500–8. doi: 10.1021/ja905929c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McFeeters RL, et al. The novel fold of scytovirin reveals a new twist for antiviral entry inhibitors. J Mol Biol. 2007;369(2):451–61. doi: 10.1016/j.jmb.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong C, et al. Potent anti-HIV activity of scytovirin domain 1 peptide. Peptides. 2006;27(7):1668–75. doi: 10.1016/j.peptides.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Xiong C, et al. Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr Purif. 2006;46(2):233–9. doi: 10.1016/j.pep.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Jensen SM, et al. Differential inhibitory effects of cyanovirin-N, griffithsin, and scytovirin on entry mediated by envelopes of gammaretroviruses and deltaretroviruses. J Virol. 2014;88(4):2327–32. doi: 10.1128/JVI.02553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garrison AR, et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antiviral Res. 2014;112:1–7. doi: 10.1016/j.antiviral.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carneiro MG, et al. Sampling of Glycan-Bound Conformers by the Anti-HIV Lectin Oscillatoria agardhii agglutinin in the Absence of Sugar. Angew Chem Int Ed Engl. 2015;54(22):6462–5. doi: 10.1002/anie.201500213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koharudin LM, Furey W, Gronenborn AM. Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin from Oscillatoria agardhii. J Biol Chem. 2011;286(2):1588–97. doi: 10.1074/jbc.M110.173278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato Y, et al. Purification and characterization of a novel lectin from a freshwater cyanobacterium, Oscillatoria agardhii. Comp Biochem Physiol B Biochem Mol Biol. 2000;125(2):169–77. doi: 10.1016/s0305-0491(99)00164-9. [DOI] [PubMed] [Google Scholar]
- 62.Sato Y, Okuyama S, Hori K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J Biol Chem. 2007;282(15):11021–9. doi: 10.1074/jbc.M701252200. [DOI] [PubMed] [Google Scholar]
- 63.Koharudin LM, et al. Structural insights into the anti-HIV activity of the Oscillatoria agardhii agglutinin homolog lectin family. J Biol Chem. 2012;287(40):33796–811. doi: 10.1074/jbc.M112.388579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitley MJ, et al. Burkholderia oklahomensis agglutinin is a canonical two-domain OAA-family lectin: structures, carbohydrate binding and anti-HIV activity. FEBS J. 2013;280(9):2056–67. doi: 10.1111/febs.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roque-Barreira MC, Campos-Neto A. Jacalin: an IgA-binding lectin. J Immunol. 1985;134(3):1740–3. [PubMed] [Google Scholar]
- 66.Bourne Y, et al. Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. Biochem J. 2002;364(Pt 1):173–80. doi: 10.1042/bj3640173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sankaranarayanan R, et al. A novel mode of carbohydrate recognition in jacalin, a Moraceae plant lectin with a beta-prism fold. Nat Struct Biol. 1996;3(7):596–603. doi: 10.1038/nsb0796-596. [DOI] [PubMed] [Google Scholar]
- 68.Jeyaprakash AA, et al. Structural basis of the carbohydrate specificities of jacalin: an X-ray and modeling study. J Mol Biol. 2003;332(1):217–28. doi: 10.1016/s0022-2836(03)00901-x. [DOI] [PubMed] [Google Scholar]
- 69.Favero J, et al. Inhibition of human immunodeficiency virus infection by the lectin jacalin and by a derived peptide showing a sequence similarity with gp120. Eur J Immunol. 1993;23(1):179–85. doi: 10.1002/eji.1830230128. [DOI] [PubMed] [Google Scholar]
- 70.Corbeau P, et al. Jacalin, a lectin with anti-HIV-1 properties, and HIV-1 gp120 envelope protein interact with distinct regions of the CD4 molecule. Mol Immunol. 1994;31(8):569–75. doi: 10.1016/0161-5890(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 71.Corbeau P, Pasquali JL, Devaux C. Jacalin, a lectin interacting with O-linked sugars and mediating protection of CD4+ cells against HIV-1, binds to the external envelope glycoprotein gp120. Immunol Lett. 1995;47(1–2):141–3. doi: 10.1016/0165-2478(95)00047-9. [DOI] [PubMed] [Google Scholar]
- 72.Koshte VL, et al. Isolation and characterization of BanLec-I, a mannoside-binding lectin from Musa paradisiac (banana) Biochem J. 1990;272(3):721–6. doi: 10.1042/bj2720721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh DD, et al. Unusual sugar specificity of banana lectin from Musa paradisiaca and its probable evolutionary origin. Crystallographic and modelling studies. Glycobiology. 2005;15(10):1025–32. doi: 10.1093/glycob/cwi087. [DOI] [PubMed] [Google Scholar]
- 74.Meagher JL, et al. Crystal structure of banana lectin reveals a novel second sugar binding site. Glycobiology. 2005;15(10):1033–42. doi: 10.1093/glycob/cwi088. [DOI] [PubMed] [Google Scholar]
- 75.Swanson MD, et al. A lectin isolated from bananas is a potent inhibitor of HIV replication. J Biol Chem. 2010;285(12):8646–55. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swanson MD, et al. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell. 2015;163(3):746–58. doi: 10.1016/j.cell.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gavrovic-Jankulovic M, et al. A novel recombinantly produced banana lectin isoform is a valuable tool for glycoproteomics and a potent modulator of the proliferation response in CD3+, CD4+, and CD8+ populations of human PBMCs. Int J Biochem Cell Biol. 2008;40(5):929–41. doi: 10.1016/j.biocel.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 78.Dimitrijevic R, et al. Recombinant banana lectin as mucosal immunostimulator. Journal of Functional Foods. 2012;4(3):636–641. [Google Scholar]
- 79.Mori T, et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280(10):9345–53. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 80.Ziolkowska NE, et al. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure. 2006;14(7):1127–35. doi: 10.1016/j.str.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nixon B, et al. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J Virol. 2013;87(11):6257–69. doi: 10.1128/JVI.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishag HZ, et al. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch Virol. 2013;158(2):349–58. doi: 10.1007/s00705-012-1489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meuleman P, et al. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob Agents Chemother. 2011;55(11):5159–67. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kouokam JC, et al. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One. 2011;6(8):e22635. doi: 10.1371/journal.pone.0022635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferir G, Palmer KE, Schols D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology. 2011;417(2):253–8. doi: 10.1016/j.virol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 86.O’Keefe BR, et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J Virol. 2010;84(5):2511–21. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Emau P, et al. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J Med Primatol. 2007;36(4–5):244–53. doi: 10.1111/j.1600-0684.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 88.Barton C, et al. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob Agents Chemother. 2014;58(1):120–7. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Keefe BR, et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci U S A. 2009;106(15):6099–104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmer KE. GRIFFITHSIN-BASED RECTAL MICROBICIDES FOR PREVENTION OF VIRAL ENTRY (PREVENT) 2016 Available from: https://projectreporter.nih.gov/project_info_description.cfm?aid=9095157&icde=0 ; Project Number: 5U19AI113182-03.
- 91.Moulaei T, et al. Griffithsin tandemers: flexible and potent lectin inhibitors of the human immunodeficiency virus. Retrovirology. 2015;12:6. doi: 10.1186/s12977-014-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hester G, et al. Structure of mannose-specific snowdrop (Galanthus nivalis) lectin is representative of a new plant lectin family. Nat Struct Biol. 1995;2(6):472–9. doi: 10.1038/nsb0695-472. [DOI] [PubMed] [Google Scholar]
- 93.Wright CS. New folds of plant lectins. Curr Opin Struct Biol. 1997;7(5):631–6. doi: 10.1016/s0959-440x(97)80071-1. [DOI] [PubMed] [Google Scholar]
- 94.Wright LM, et al. Purification, crystallization and preliminary X-ray analysis of a mannose-binding lectin from bluebell (Scilla campanulata) bulbs. Acta Crystallogr D Biol Crystallogr. 1996;52(Pt 5):1021–3. doi: 10.1107/S0907444996006889. [DOI] [PubMed] [Google Scholar]
- 95.Wright LM, et al. Isolation, characterization, molecular cloning and molecular modelling of two lectins of different specificities from bluebell (Scilla campanulata) bulbs. Biochem J. 1999;340( Pt 1):299–308. [PMC free article] [PubMed] [Google Scholar]
- 96.Kaku H, et al. Carbohydrate-binding specificity of the daffodil (Narcissus pseudonarcissus) and amaryllis (Hippeastrum hybr.) bulb lectins. Arch Biochem Biophys. 1990;279(2):298–304. doi: 10.1016/0003-9861(90)90495-k. [DOI] [PubMed] [Google Scholar]
- 97.Sauerborn MK, et al. Insights into carbohydrate recognition by Narcissus pseudonarcissus lectin: the crystal structure at 2 A resolution in complex with alpha1–3 mannobiose. J Mol Biol. 1999;290(1):185–99. doi: 10.1006/jmbi.1999.2862. [DOI] [PubMed] [Google Scholar]
- 98.Marchetti M, et al. Inhibition of herpes simplex, rabies and rubella viruses by lectins with different specificities. Res Virol. 1995;146(3):211–5. doi: 10.1016/0923-2516(96)80581-4. [DOI] [PubMed] [Google Scholar]
- 99.Balzarini J, et al. Alpha-(1–3)- and alpha-(1–6)-D-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob Agents Chemother. 1991;35(3):410–6. [Google Scholar]
- 100.Weiler BE, et al. Sulphoevernan, a polyanionic polysaccharide, and the narcissus lectin potently inhibit human immunodeficiency virus infection by binding to viral envelope protein. J Gen Virol. 1990;71( Pt 9):1957–63. doi: 10.1099/0022-1317-71-9-1957. [DOI] [PubMed] [Google Scholar]
- 101.Shibuya N, et al. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem. 1988;263(2):728–34. [PubMed] [Google Scholar]
- 102.Wright CS, Kaku H, Goldstein IJ. Crystallization and preliminary X-ray diffraction results of snowdrop (Galanthus nivalis) lectin. J Biol Chem. 1990;265(3):1676–7. [PubMed] [Google Scholar]
- 103.Hester G, Wright CS. The mannose-specific bulb lectin from Galanthus nivalis (snowdrop) binds mono- and dimannosides at distinct sites. Structure analysis of refined complexes at 2.3 A and 3.0 A resolution. J Mol Biol. 1996;262(4):516–31. doi: 10.1006/jmbi.1996.0532. [DOI] [PubMed] [Google Scholar]
- 104.Wright CS, Hester G. The 2.0 A structure of a cross-linked complex between snowdrop lectin and a branched mannopentaose: evidence for two unique binding modes. Structure. 1996;4(11):1339–52. doi: 10.1016/s0969-2126(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 105.Balzarini J, et al. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother. 2004;48(10):3858–70. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoorelbeke B, et al. HIV-1 envelope trimer has similar binding characteristics for carbohydrate-binding agents as monomeric gp120. FEBS Lett. 2013;587(7):860–6. doi: 10.1016/j.febslet.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 107.Chantalat L, et al. X-ray structure solution of amaryllis lectin by molecular replacement with only 4% of the total diffracting matter. Acta Crystallogr D Biol Crystallogr. 1996;52(Pt 6):1146–52. doi: 10.1107/S090744499600546X. [DOI] [PubMed] [Google Scholar]
- 108.An J, et al. Anti-HIV I/II activity and molecular cloning of a novel mannose/sialic acid-binding lectin from rhizome of Polygonatum cyrtonema Hua. Acta Biochim Biophys Sin (Shanghai) 2006;38(2):70–8. doi: 10.1111/j.1745-7270.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 109.Ding J, et al. Crystal structures of a novel anti-HIV mannose-binding lectin from Polygonatum cyrtonema Hua with unique ligand-binding property and super-structure. J Struct Biol. 2010;171(3):309–17. doi: 10.1016/j.jsb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 110.Sumner JB, Howell SF, Zeissig A. Concanavalin a and Hemagglutination. Science. 1935;82(2116):65–6. doi: 10.1126/science.82.2116.65. [DOI] [PubMed] [Google Scholar]
- 111.Sawyer WH. Self-association, conformation and binding equilibria of concanavalin A. Adv Exp Med Biol. 1975;55:71–95. doi: 10.1007/978-1-4684-0949-9_5. [DOI] [PubMed] [Google Scholar]
- 112.Hardman KD, Ainsworth CF. Structure of concanavalin A at 2.4-A resolution. Biochemistry. 1972;11(26):4910–9. doi: 10.1021/bi00776a006. [DOI] [PubMed] [Google Scholar]
- 113.Naismith JH, et al. Refined structure of concanavalin A complexed with methyl alpha-D-mannopyranoside at 2.0 A resolution and comparison with the saccharide-free structure. Acta Crystallogr D Biol Crystallogr. 1994;50(Pt 6):847–58. doi: 10.1107/S0907444994005287. [DOI] [PubMed] [Google Scholar]
- 114.Okada Y, Kim J. Interaction of concanavalin A with enveloped viruses and host cells. Virology. 1972;50(2):507–15. doi: 10.1016/0042-6822(72)90401-1. [DOI] [PubMed] [Google Scholar]
- 115.Kornfeld R, Ferris C. Interaction of immunoglobulin glycopeptides with concanavalin A. J Biol Chem. 1975;250(7):2614–9. [PubMed] [Google Scholar]
- 116.Pashov A, et al. Concanavalin A binding to HIV envelope protein is less sensitive to mutations in glycosylation sites than monoclonal antibody 2G12. Glycobiology. 2005;15(10):994–1001. doi: 10.1093/glycob/cwi083. [DOI] [PubMed] [Google Scholar]
- 117.Shibuya N, et al. Carbohydrate binding properties of the stinging nettle (Urtica dioica) rhizome lectin. Arch Biochem Biophys. 1986;249(1):215–24. doi: 10.1016/0003-9861(86)90577-1. [DOI] [PubMed] [Google Scholar]
- 118.Wagner H, Willer F, Kreher B. Biologically active compounds from the aqueous extract of Urtica dioica. Planta Med. 1989;55(5):452–4. doi: 10.1055/s-2006-962062. [DOI] [PubMed] [Google Scholar]
- 119.Harata K, Muraki M. Crystal structures of Urtica dioica agglutinin and its complex with tri-N-acetylchitotriose. J Mol Biol. 2000;297(3):673–81. doi: 10.1006/jmbi.2000.3594. [DOI] [PubMed] [Google Scholar]
- 120.Saul FA, et al. Crystal structure of Urtica dioica agglutinin, a superantigen presented by MHC molecules of class I and class II. Structure. 2000;8(6):593–603. doi: 10.1016/s0969-2126(00)00142-8. [DOI] [PubMed] [Google Scholar]
- 121.Balzarini J, et al. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 1992;18(2):191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 122.Keyaerts E, et al. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75(3):179–87. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Day CW, et al. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. 2009;395(2):210–22. doi: 10.1016/j.virol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Charan RD, et al. Isolation and characterization of Myrianthus holstii lectin, a potent HIV-1 inhibitory protein from the plant Myrianthus holstii(1) J Nat Prod. 2000;63(8):1170–4. doi: 10.1021/np000039h. [DOI] [PubMed] [Google Scholar]
- 125.Chen Y, et al. Jasmonic acid methyl ester induces the synthesis of a cytoplasmic/nuclear chito-oligosaccharide binding lectin in tobacco leaves. FASEB J. 2002;16(8):905–7. doi: 10.1096/fj.01-0598fje. [DOI] [PubMed] [Google Scholar]
- 126.Gordts SC, et al. NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J Antimicrob Chemother. 2015;70(6):1674–85. doi: 10.1093/jac/dkv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mikheyskaya LV, et al. Isolation and Characterization of a New Beta-Galactose-Specific Lectin from the Sea Worm Chaetopterus-Variopedatus. Carbohydrate Research. 1995;275(1):193–200. [Google Scholar]
- 128.Wang JH, et al. A beta-galactose-specific lectin isolated from the marine worm Chaetopterus variopedatus possesses anti-HIV-1 activity. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142(1–2):111–7. doi: 10.1016/j.cbpc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 129.Molchanova V, et al. A new lectin from the sea worm Serpula vermicularis: isolation, characterization and anti-HIV activity. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145(2):184–93. doi: 10.1016/j.cbpc.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 130.Luk’yanov PA, et al. Carbohydrate-binding proteins of marine invertebrates. Russian Journal of Bioorganic Chemistry. 2007;33(1):161–169. [PubMed] [Google Scholar]
- 131.Belogortseva NI, et al. Isolation and characterization of new GalNAc/Gal-specific lectin from the sea mussel Crenomytilus grayanus. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(1):45–50. doi: 10.1016/s0742-8413(97)00180-1. [DOI] [PubMed] [Google Scholar]
- 132.Jakob M, et al. Structure of a lectin from the sea mussel Crenomytilus grayanus (CGL) Acta Crystallogr F Struct Biol Commun. 2015;71(Pt 11):1429–36. doi: 10.1107/S2053230X15019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liao JH, et al. A Multivalent Marine Lectin from Crenomytilus grayanus Possesses Anti-cancer Activity through Recognizing Globotriose Gb3. J Am Chem Soc. 2016;138(14):4787–95. doi: 10.1021/jacs.6b00111. [DOI] [PubMed] [Google Scholar]
- 134.Furtak VA, et al. Cellular localization of mucin type receptors determined with novel GalNac/Gal-specific lectin from sea mussel Crenomytilus grayanus in human colon tumors. Biull Eksp Biol Med. 1999;128(10):441–4. [PubMed] [Google Scholar]
- 135.Kovalchuk SN, et al. cDNA cloning and structural characterization of a lectin from the mussel Crenomytilus grayanus with a unique amino acid sequence and antibacterial activity. Fish Shellfish Immunol. 2013;35(4):1320–4. doi: 10.1016/j.fsi.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 136.Chikalovets IV, et al. A lectin with antifungal activity from the mussel Crenomytilus grayanus. Fish Shellfish Immunol. 2015;42(2):503–7. doi: 10.1016/j.fsi.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 137.Belogortseva NI, et al. Isolation and Characterization of a Structurally Unusual Lectin from Ascidian Didemnum-Ternatum (Dtl) Bioorganicheskaya Khimiya. 1994;20(8–9):975–983. [PubMed] [Google Scholar]
- 138.Kljajic Z, et al. A D-mannose-specific lectin from Gerardia savaglia that inhibits nucleocytoplasmic transport of mRNA. Eur J Biochem. 1987;169(1):97–104. doi: 10.1111/j.1432-1033.1987.tb13585.x. [DOI] [PubMed] [Google Scholar]
- 139.Muller WE, et al. The D-mannose-specific lectin from Gerardia savaglia blocks binding of human immunodeficiency virus type I to H9 cells and human lymphocytes in vitro. J Acquir Immune Defic Syndr. 1988;1(5):453–8. [PubMed] [Google Scholar]
- 140.Moncla BJ, et al. Degradation of naturally occurring and engineered antimicrobial peptides by proteases. Adv Biosci Biotechnol. 2011;2(6):404–408. doi: 10.4236/abb.2011.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nicolson GL. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- 142.Baker MP, et al. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self Nonself. 2010;1(4):314–322. doi: 10.4161/self.1.4.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wallace MS, et al. Intrathecal ziconotide in the treatment of chronic nonmalignant pain: a randomized, double-blind, placebo-controlled clinical trial. Neuromodulation. 2006;9(2):75–86. doi: 10.1111/j.1525-1403.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 144.Wysham CH, MacConell L, Hardy E. Efficacy and Safety of Multiple Doses of Exenatide Once-Monthly Suspension in Patients With Type 2 Diabetes: A Phase II Randomized Clinical Trial. Diabetes Care. 2016;39(10):1768–76. doi: 10.2337/dc16-0238. [DOI] [PubMed] [Google Scholar]
- 145.Brin MF. Basic and clinical aspects of BOTOX. Toxicon. 2009;54(5):676–82. doi: 10.1016/j.toxicon.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 146.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90(1):196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Knolle PA, et al. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology. 1996;24(4):824–9. doi: 10.1002/hep.510240413. [DOI] [PubMed] [Google Scholar]
- 148.Kouokam JC, Lasnik AB, Palmer KE. Studies in a Murine Model Confirm the Safety of Griffithsin and Advocate Its Further Development as a Microbicide Targeting HIV-1 and Other Enveloped Viruses. Viruses. 2016;8(11) doi: 10.3390/v8110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smee DF, et al. Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin-N. Antiviral Res. 2008;80(3):266–71. doi: 10.1016/j.antiviral.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamamoto HS, Xu Q, Fichorova RN. Homeostatic properties of Lactobacillus jensenii engineered as a live vaginal anti-HIV microbicide. BMC Microbiol. 2013;13:4. doi: 10.1186/1471-2180-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li M, et al. Incorporation of the HIV-1 microbicide cyanovirin-N in a food product. J Acquir Immune Defic Syndr. 2011;58(4):379–84. doi: 10.1097/QAI.0b013e31823643fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sexton A, et al. Transgenic plant production of Cyanovirin-N, an HIV microbicide. FASEB J. 2006;20(2):356–8. doi: 10.1096/fj.05-4742fje. [DOI] [PubMed] [Google Scholar]
- 153.O’Keefe BR, et al. Engineering soya bean seeds as a scalable platform to produce cyanovirin-N, a non-ARV microbicide against HIV. Plant Biotechnol J. 2015;13(7):884–92. doi: 10.1111/pbi.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vamvaka E, et al. Cyanovirin-N produced in rice endosperm offers effective pre-exposure prophylaxis against HIV-1BaL infection in vitro. Plant Cell Reports. 2016;35:1309–1319. doi: 10.1007/s00299-016-1963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vamvaka E, et al. Rice endosperm is cost-effective for the production of recombinant griffithsin with potent activity against HIV. Plant Biotechnol J. 2016;14:1427–1437. doi: 10.1111/pbi.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Q, Davis KR. The potential of plants as a system for the development and production of human biologics. F1000Res. 2016 May 19;(5) doi: 10.12688/f1000research.8010.1. pii: F1000 FacultyRev-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zappe H, Snell ME, Bossard MJ. PEGylation of cyanovirin-N, an entry inhibitor of HIV. Adv Drug Deliv Rev. 2008;60(1):79–87. doi: 10.1016/j.addr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 158.Takahashi A, et al. The high mannose-type glycan binding lectin actinohivin: dimerization greatly improves anti-HIV activity. J Antibiot (Tokyo) 2011;64(8):551–7. doi: 10.1038/ja.2011.51. [DOI] [PubMed] [Google Scholar]