Abstract
Importance
A combined objective and subjective wireless monitoring program of patient-centered outcomes can be carried out in patients before and after major abdominal surgery.
Objective
To conduct a proof-of-concept study of a wireless, patient-centered outcomes monitoring program before and after major abdominal cancer surgery.
Design
Patients wore wristband pedometers and completed online patient-reported outcome surveys (symptoms, QOL) 3 to 7 days before surgery, through hospitalization, and for two weeks post-discharge. Alerts were generated for all moderate to severe scores for symptoms and QOL. Surgery-related data was collected via electronic medical chart and complications were calculated using the Clavien-Dindo classification.
Setting
The study was carried out in the inpatient and outpatient surgical oncology unit of one NCI designated comprehensive cancer center.
Participants
Eligible patients were scheduled to undergo curative resection for hepatobiliary and gastrointestinal malignancies, English-speaking, and 18 years or older. Twenty participants were enrolled over 4 months.
Main Outcomes and Measures
Outcomes included 1) adherence with wearing the pedometer; 2) adherence with completing the surveys (MDASI and EQ-5D-5L), and 3) satisfaction with the monitoring program.
Results
Pedometer adherence (88% pre-op versus 83% post-discharge) was higher than survey adherence (75% completed). The median number of steps at day 7 was 1689 (19% of steps at baseline). This correlated with the comprehensive complication index (CCI), for which the median was 15/100 (r = −0.64, p<0.05). Post-discharge overall symptom severity (2.3/10) and symptom interference with activities (3.5/10) were mild. Pain, fatigue, and appetite loss were moderate after surgery (4.4, 4.7, 4.0). QOL scores were lowest at discharge (66.6/100), but improved at week 2 (73.9/100). While patient-reported outcomes returned to baseline at 2 weeks, the number of steps was only one third of pre-operative baseline.
Conclusions and Relevance
Wireless monitoring of combined subjective and objective patient-centered outcomes can be carried out in the surgical oncology setting. Pre- and post-operative patient-centered outcomes have the potential of identifying high risk populations who may need additional interventions to support postoperative functional and symptom recovery.
Introduction
Major abdominal surgeries for gastrointestinal (GI) malignancies, are complex and at higher risk for post-operative complications. This can result in prolonged hospital stay, decreased functional status, and poor quality of life (QOL).1–5 Recent changes in the healthcare system have focused attention on integrating patient-centeredness into surgical oncology.6 The Institute of Medicine (IOM) defines patient-centeredness as care that is respectful and responsive to patient preferences, needs, and values.7 A common approach to integrating this concept is to monitor patient-centered outcomes, which are defined as any health-related data created, recorded, gathered, or inferred by or from patients/caregivers to address a health concern.8
Functional status is an important aspect of surgical care, and is often used to guide clinical decisions before and after surgery. Physical and functional limitations are common after major abdominal cancer surgery.9,10 Moreover, preliminary evidence suggests that functional status is a potential predictor of traditional surgical outcomes, such as post-operative complications, length of hospital stay, and readmissions.3,5,11–16 Patient-centered outcomes, including symptoms and QOL, are of major importance in abdominal surgery because of the high-risk of post-operative compilations and their potential impact.17,18 Indeed, QOL after abdominal procedures decreases considerably in the early postoperative phase, and that full recovery is achieved up to 6 months after surgery.2,4,15,19
Technological advances with wearable devices and sensors enable the monitoring of patient’s longitudinal cancer experience.8 Pedometers that capture data on daily steps are an increasingly popular method of assessing functional status in research and clinical care, as they are objective and relatively inexpensive.20 Moreover, steps data can potentially serve as a surrogate of mobility, speed of recovery. A recent systematic review of 22 studies concluded that the validity and reliability of pedometers were generally high for measuring daily steps.21
To date, patient-centered outcomes are not routinely captured and monitored in the peri-operative setting. The purpose of this study was 1) to determine whether a wireless, patient-centered outcomes monitoring program can be carried out for cancer patients undergoing a major abdominal procedure; 2) to describe the trajectory and trends of functional recovery (daily steps), symptoms, and QOL from before surgery to 2 weeks post-discharge; and 3) to determine whether an alert system can be carried out when deviations from pre-determined outcome scores occur. We hypothesized that the program’s subjective and objective measures can shed light on the “black box” of patient-centered outcomes and functional status during postoperative recovery at home.
Methods
Sample and Setting
Patients scheduled to undergo surgery for the treatment of GI and hepatobiliary malignancies (gastric, colorectal, liver, pancreas), English-speaking, and 18 years or older were eligible for participation in the study. All eligible patients who met the study inclusion criteria were identified and recruited from the surgical oncology ambulatory clinics of one NCI-designated comprehensive cancer center between April 2015 and July 2016. Study procedures and protocol were approved by the Institutional Review Board, and all participating patients provided informed consent prior to enrollment.
Patient-Centered Outcomes
Following informed consent, patients were given a commercially-available wristband pedometer device, the Vivofit 2® (Garmin Company), to monitor the number of daily steps as an objective, patient-generated measure of functional recovery. Steps data were continuously collected 3 to 7 days before surgery, during hospitalization, and up to two weeks post-discharge.
Patient-reported symptoms and QOL were assessed via an online system designed using DatStat (Seattle, WA). The system sends personalized links to the online surveys via a patient-provided email address. The survey contained 19 questions and included two validated measures. Symptom severity and interference with activities were assessed using the MD Anderson Symptom Inventory (MDASI), a validated measure of 13 common cancer-related symptoms as rated on a 10-point scale.22 QOL and general health status was assessed using the EQ-5D-5L. This validated tool evaluates five QOL variables: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. One final item evaluates overall health state using a visual analogue scale with the end points labeled “best to worst imaginable” health state (0–100 numeric value). The EQ-5D-5L has been widely used in clinical trials and also used in quality-adjusted survival analysis.23–26
Clinical/Surgical Outcomes
Relevant clinical/surgical data were obtained via electronic medical chart (EMR). These included primary diagnosis, co-morbidities, surgery date, procedure type, surgical technique (open, laparoscopic, robotic-assisted), ASA classification, length of hospital stay, and readmissions. The patient’s pre-operative performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) score, ranging from 0 to 5, where 0 denotes an absence of symptoms and 5 denoting death. Post-operative complications were calculated using the comprehensive complications index (CCI) based on the Clavien-Dindo classification.27,28 The CCI is a score ranging from 0 to 100 that takes into account all 30 day complications and their treatment, where 0 indicates no deviation from the expected post-operative course and 100 is death.
Data Integration and Study Procedures
Steps data were automatically and wirelessly synchronized via smartphone, tablets, and computers application. With permission, patient’s steps data was shared with the research team via a secure group account created through an online system. Synchronized steps data were integrated into the study database via data capture procedures per manufacturer instructions. Patient-reported symptoms and QOL obtained via the online survey system were stored electronically in encrypted, password-protected, secure computers that met all HIPAA requirements.
Online symptoms and QOL surveys were completed once before surgery (baseline) and before hospital discharge. After discharge, patients were followed for two weeks. During this time, they completed online symptom assessment three times per week (post-discharge days 1, 3, 5, 8, 10, 12) and QOL assessment once per week (days 5 and 12). Patients completed a brief satisfaction survey that assessed monitoring acceptability. Every instance of patient completion of the online survey was computed as an “encounter” and the total number of encounters was monitored.
Feedback system
An email alert to research staff was automatically generated within one minute of survey completion for all moderate to severe scores for symptoms and QOL. This pre-determined threshold prompted a telephone call from the research staff to the patient for further status assessment as well as notification to the surgical team. Every instance of telephone assessment was computed as an “encounter” and the total number of encounters was monitored.
Statistical Analysis
Data from the DatStat web-based system we designed were audited for accuracy prior to analysis. Data was summarized using means for normally distributed continuous data, medians for non-normally distributed continuous data, and proportions and percentages for categorical data. A correlation coefficient was calculated between the number of steps at day 7 and post-operative complications as calculated by the CCI. Established instruments were scored according to standard instructions, and appropriate descriptive statistics were computed. Outcomes were calculated for the percentage of patients who were able to complete the 1) MDASI instruments and 2) the EQ-5D-5L instruments after discharge, and the percentage of patients who were able to wear the Vivofit 2 pedometer. Patient-reported satisfaction with the monitoring program was also assessed.
Results
A total of 29 eligible patients were invited to participate in the study. Of those, 21 (80%) patients agreed to participate and provided written informed consent. The most common reasons patients gave for declining participation was being too busy or too overwhelmed. One consented patient had emergency surgery and discontinued study participation. This yielded a final sample of 20 patients with evaluable data.
Study findings revealed that 88% of patients wore the pedometer device for at least three days before surgery (median=6 days), while 88% wore the device for at least three days during hospitalization (median=6 days), and 83% wore the device for at least one week after discharge (median=15 days). For electronic symptom assessment, 65% completed the eight evaluation time points. For QOL, 75% completed the four evaluation time points. It took an average of seven minutes to complete the MDASI, and four minutes for the EQ-5D-5L.
For satisfaction, patients reported that the wearable device (median=5, range of 2–5; 0=not satisfied to 5=extremely satisfied) and online survey system (median 4, range 2–5) were easy to use. Three patients had difficulties with the devices; two of them lost the device and another a malfunctioning battery. Patients reported no difficulties with answering survey questions (95%), and the majority found that the length of time was just right for completing online surveys (95%) and using the device (70%). About 25% of patients thought that the length of time for using the wearable device was too short.
Sociodemographic and Clinical Characteristics
Table 1 provides the sociodemographic and clinical characteristics of the 20 patients enrolled in this study. Mean age was 55, and three-quarters were female (75%), Caucasian (65%), and married (60%). Subdivision by site revealed 30% colorectal, 30% pancreas, 30% liver, and 10% gastric resections. The median number of self-reported co-morbidities was 2 (range 0–7), and the majority of co-morbid conditions were cardiovascular (40%). Forty percent of the procedures were minimally-invasive. Median length of hospital stay was 6 days. Two patients (10%) were discharged to a skilled nursing/rehabilitation facility, and two were readmitted at 30 days for symptom management.
Table 1.
Sociodemographic and Clinical Characteristics (N=20)
| N (%) | |
|---|---|
| Age | |
|
| |
| Median (Range) | 55.5 (22 – 74) |
|
| |
| Gender | |
|
| |
| Female | 15 (75) |
| Male | 5 (25) |
|
| |
| Race | |
|
| |
| White | 13 (65) |
| Black/African American | 2 (10) |
| Asian | 3 (15) |
| Native Indian or Alaska Native | 1 (5) |
| Other | 1 (5) |
|
| |
| Ethnicity | |
|
| |
| Non-Hispanic | 17 (85) |
| Hispanic or Latino | 3 (15) |
|
| |
| Marital Status | |
|
| |
| Married | 12 (60) |
| Divorced | 5 (25) |
| Never married | 2 (10) |
| Domestic partnership | 1 (5) |
|
| |
| Living Situation | |
|
| |
| Spouse or Spouse/Children | 12 (60) |
| Alone | 4 (20) |
| Adult Children/Friend | 3 (15) |
| Parents | 1 (5) |
|
| |
| Education | |
|
| |
| High school graduate/GED | 2 (10) |
| Associate degree/some college | 6 (30) |
| Vocational/technical school | 3 (15) |
| Bachelors degree | 5 (25) |
| Advanced degree | 4 (20) |
|
| |
| Employment Status | |
|
| |
| Employed 32 hours or more per week | 8 (40) |
| Employed less than 32 hours per week | 1 (5) |
| Retired | 5 (25) |
| Homemaker | 1 (5) |
| Disabled | 3 (15) |
| Unemployed | 2 (10) |
|
| |
| Distance Traveled for Cancer Care | |
|
| |
| Less than 5 miles | 1 (5) |
| 5 to 10 miles | 1 (5) |
| 11 to 15 miles | 2 (10) |
| More than 15 miles | 16 (80) |
|
| |
| Resection Site | |
|
| |
| Gastric | 2 (10) |
| Colorectal | 6 (30) |
| Pancreas | 6 (30) |
| Liver | 6 (30) |
|
| |
| ECOG Performance Scale | |
|
| |
| 0 | 13 (65%) |
| 1 | 7 (35%) |
|
| |
| ASA Classification | |
|
| |
| II | 2 (10) |
| III | 16 (80) |
| IV | 2 (10) |
|
| |
| Self-Reported Number of Co-Morbidities | |
|
| |
| Median (Range) | 2 (0–7) |
|
| |
| Type of Co-Morbidities | |
|
| |
| Cardiovascular | 8 (40) |
| Diabetes | 7 (35) |
| None | 5 (25) |
|
| |
| Surgery Technique | |
|
| |
| Open | 12 (60) |
| Minimally Invasive (Laparoscopic, Robotic-Assisted) | 6 (40) |
|
| |
| Pre-Operative Treatments | |
|
| |
| Yes | 2 (10) |
|
| |
| Length of Hospital Stay | |
|
| |
| Median (Range) | 6 (1 – 13) |
|
| |
| Comprehensive Complications Index (CCI) | |
|
| |
| Median (interquartile range) | 15 (0–22.6) |
|
| |
| Readmission within 30 days | |
|
| |
| Yes | 2 (10%) |
Daily Steps Trajectory and Trends
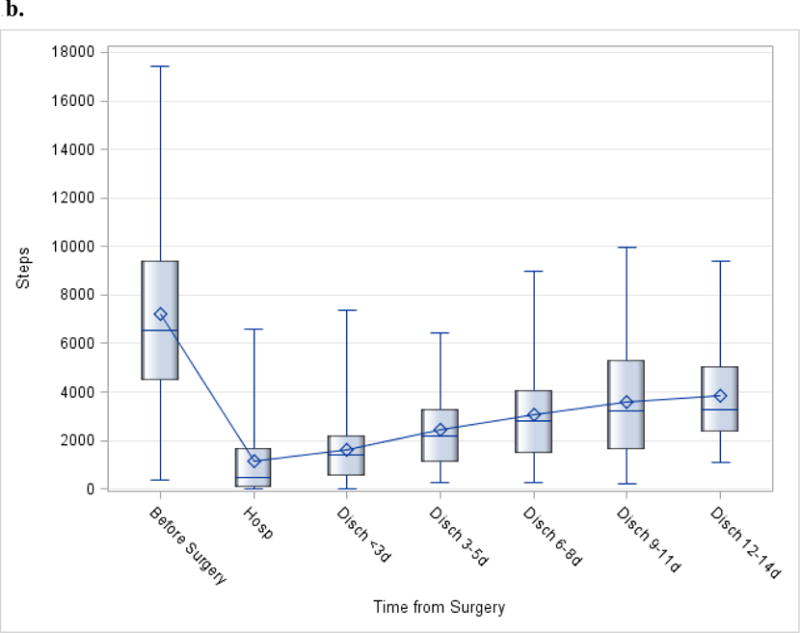
The median number of steps per day before surgery was 6,562; this number decreased to 482 during hospitalization, and increased to 2,483 in the first 2 weeks post-discharge (Figure 1a). Examination of functional status and daily steps from discharge to two weeks post-discharge revealed that the average and median steps were lowest during hospitalization (Figure 1a–b). The number of daily steps steadily increased every three days after discharge, until day 14, when it appeared to level off (Figure 1b). The median CCI score was 15 (out of 100, Table 1). The median number of steps at day 7 was 1689 (19% of steps at baseline, Figure 1a). This correlated with CCI score (r=−0.64, p<0.05), where patients with fewer daily steps had a higher CCI score (Figure 2).
Figure 1.
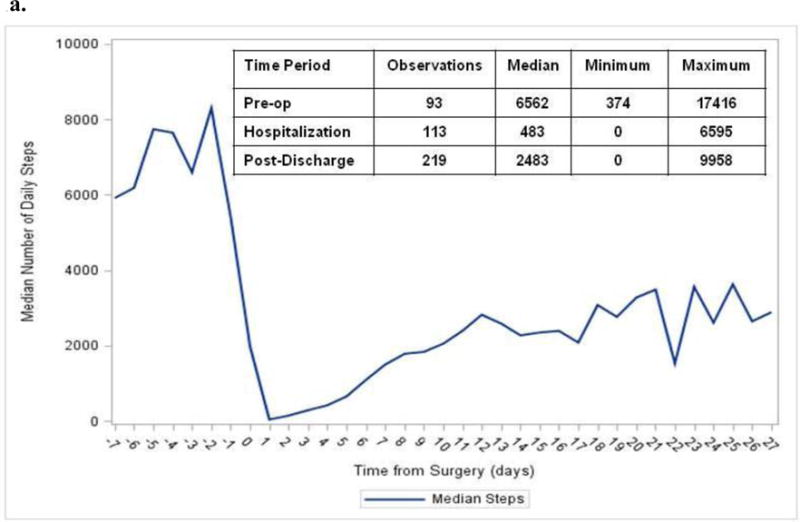
a. Functional Status Trajectory and Trends. A. Median daily steps measured before surgery (pre-op), during hospitalization, and during recovery period (post-discharge) using Vivofit 2.
b. Trends for distribution of daily steps before surgery (pre-op), hospitalization, and recovery (post-discharge). The box-and-whiskers plot displays the mean (diamond), quartiles, and minimum and maximum observations at each time-point.
Figure 2.
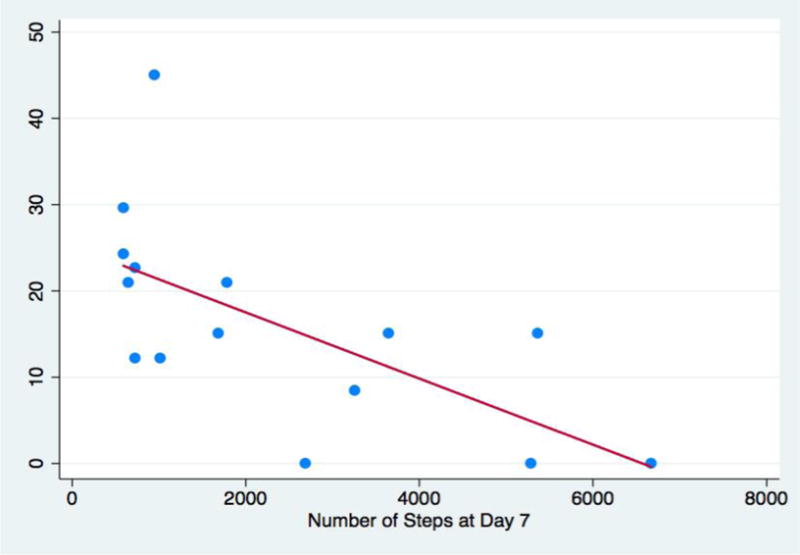
Association between Comprehensive Complication Index (CCI) and daily steps at post-op day 7. Scale of 0–100; 0=no deviation from expected post-operative course, 100=death.
CCI median = 15 (IQR: 0–22.6)
Symptoms and Quality of Life Trajectory
Figure 3 depicts findings on the trajectory of symptom, symptom interference with activities and QOL, collected before surgery up to two weeks post-discharge. Overall symptom severity and interference scores were worse at discharge and week 1 post-discharge (days 1, 3, and 5), with gradual improvements at week 2. Similarly, overall QOL/general health status scores were lowest at discharge and week 1 post-discharge, but improved at week 2.
Figure 3.
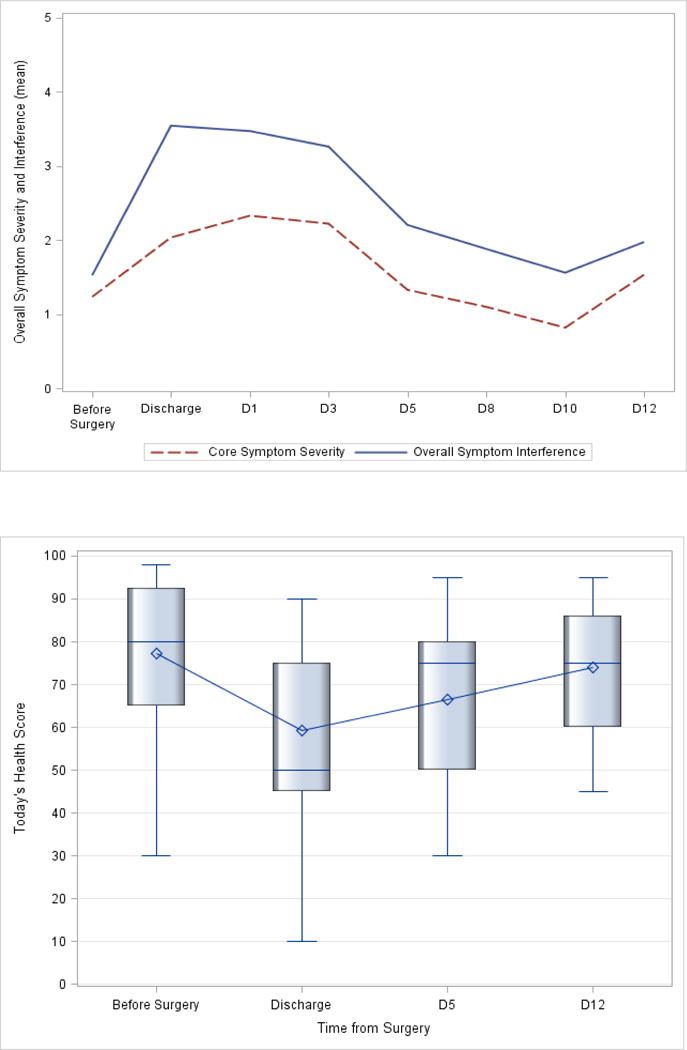
Trajectory of symptom severity, symptom interference with activities 0–10 scale; higher=worse (A), and quality of life - today’s health (B), before surgery and up to two weeks post-discharge. The box-and-whiskers plot displays the mean (diamond), quartiles, and minimum and maximum observations at each time-point.
Table 2 presents the trajectory of mean scores for individual symptom and QOL dimension items. Patients reported moderate severity for fatigue and pain (score of 4 or >) at 1 week post-discharge, with gradual improvements to mild severity at week 2. For QOL dimensions, patients reported moderate problems with usual activities at discharge.
Table 2.
Symptoms and quality of life scores over time (Mean, SD).
| Before Surgery | Discharge | Day 1 | Day 3 | Day 5 | Day 8 | Day 10 | Day 12 | |
|---|---|---|---|---|---|---|---|---|
| Overall Symptom Scores (0–10 scale, higher=worse) | ||||||||
|
| ||||||||
| Symptom Interference | 1.5±1.9 | 3.5±2.7 | 3.5±1.9 | 3.3±1.9 | 2.2±1.9 | 1.9±1.7 | 1.6±1.0 | 2.0±1.8 |
| Symptom Severity | 1.2±1.2 | 2.0±1.4 | 2.3±1.5 | 2.2±1.5 | 1.3±0.9 | 1.1±1.0 | 0.8±0.6 | 1.5±1.5 |
|
| ||||||||
| Individual Symptom Scores (0–10 scale, higher=worse) | ||||||||
|
| ||||||||
| Fatigue | 3.1±2.4 | 1.7±2.7 | 4.7±2.0 | 4.5±2.0 | 3.3±2.4 | 2.4±2.1 | 1.8±1.6 | 3.2±2.6 |
| Pain | 2.2±3.1 | 1.5±3.2 | 4.4±3.2 | 4.0±3.1 | 2.9±2.4 | 2.6±2.8 | 1.9±1.2 | 2.8±2.0 |
| Appetite | 0.4±1.1 | 2.6±2.7 | 4.0±2.8 | 3.7±2.8 | 2.6±2.8 | 2.1±2.6 | 1.9±2.1 | 2.5±2.9 |
| Sleep | 2.5±2.9 | 3.2±3.0 | 3.6±2.6 | 3.4±2.5 | 2.2±2.3 | 2.0±2.1 | 1.1±1.6 | 2.5±2.9 |
| Drowsy | 2.1±2.1 | 3.2±2.4 | 3.5±2.3 | 3.2±2.3 | 2.0±2.0 | 1.6±2.1 | 1.2±1.3 | 2.3±2.6 |
| Dry mouth | 1.0±1.9 | 3.4±3.4 | 2.7±3.6 | 2.7±3.5 | 0.7±0.9 | 0.4±0.5 | 0.5±0.8 | 1.8±2.7 |
| Upset | 1.5±2.4 | 0.9±1.6 | 2.2±3.0 | 2.1±2.9 | 1.2±2.1 | 0.7±1.5 | 0.5±1.0 | 0.7±1.5 |
| Sad | 1.4±2.3 | 1.2±2.4 | 2.1±2.7 | 2.1±2.6 | 0.4±0.9 | 0.1±0.3 | 0.3±0.5 | 0.6±1.4 |
| Dyspnea | 0.8±1.4 | 1.1±1.5 | 1.2±1.3 | 1.0±1.3 | 0.5±1.1 | 0.4±0.8 | 0.5±0.9 | 0.8±1.0 |
| Memory | 0.4±0.9 | 1.0±1.5 | 0.9±1.6 | 1.1±1.7 | 0.5±0.8 | 0.4±0.5 | 0.4±0.5 | 0.7±1.7 |
| Nausea | 0.5±1.2 | 0.5±0.9 | 0.7±1.2 | 0.8±1.3 | 0.5±1.0 | 1.0±2.0 | 0.2±0.4 | 0.6±0.9 |
|
| ||||||||
| Quality of Life Health Dimensions a (0–5 scale, higher=more problems) | ||||||||
|
| ||||||||
| Mobility | 0.2±0.4 | 1.6±0.7 | – | – | 0.9±0.7 | – | – | 0.6±0.5 |
| Self-Care | 0.0 | 1.7±1.1 | – | – | 0.7±0.7 | – | – | 0.1±0.4 |
| Usual Activity | 0.4±0.7 | 2.6±1.2 | – | – | 1.4±0.9 | – | – | 1.0±0.6 |
| Pain/Discomfort | 0.8±0.9 | 1.8±1.0 | – | – | 1.2±0.7 | – | – | 1.0±0.5 |
| Anxiety/Depression | 0.5±0.8 | 0.4±0.6 | – | – | 0.3±0.5 | – | – | 0.1±0.4 |
|
| ||||||||
| General Health Status (0–100 scale, higher=better health) | ||||||||
|
| ||||||||
| Today’s Health | 77.2±18.3 | 59.3±23.5 | – | – | 66.6±19.7 | – | – | 73.9±15.8 |
Assessed at baseline, discharge, day 5, and day 12 only
Feedback System Encounters
Of the 160 encounters throughout the study (defined as the number of times patients completed the online surveys), 54 (33%) generated an email notification to the research staff. Of those 39 (72%) telephonic encounters were for symptoms. The most commonly reported symptom was pain and patient often reported not taking the prescribed medication. Most of the alerts were generated during the first week and then decreased during the second week of monitoring.
Discussion
The present study found that a wireless, patient-centered outcomes monitoring program that incorporated subjective and objective measures of recovery can be carried out in cancer patients undergoing major abdominal surgery. Routine monitoring of patient-centered outcomes have predominantly been tested in chemotherapy settings29,30, with positive effects on patient-provider communication,31–34 detecting unrecognized problems,30,34–37 guiding clinical care,36,38–40 and improving health outcomes.40–44 In transplant patients, changes in symptom intensity and global physical health were all significantly associated with changes in average daily steps, as measured by wearable pedometers.20 Although these data are promising, the current study is the one of the first to shed light on integrating wireless monitoring of patient-centered outcomes and functional recovery in a surgical oncology population.
Adherence to wearing the wristband pedometer was acceptable, and patients were satisfied with the wireless monitoring program. The response rates to the online survey of 65% to 75% are similar to others reported in surgical populations. A pilot study in colorectal cancer surgery using tablets to assess symptoms and QOL reported a slightly lower adherence rate (63%).45 Measurement of step numbers using commercially available pedometers serves as an objective metric of patient’s functional status and recovery. These allow real-time efficient, unobtrusive monitoring regardless of patient’s cognition, literacy, language, or health status. Moreover, they complement and augment subjective reports of symptoms and QOL, and are typically low cost and comfortable long-term.20 To our knowledge, we are among the first and few to combine real-time monitoring of both objective functional status and subjective patient-reported symptoms and QOL.
Our analysis of mobility revealed that, as expected, an abrupt decrease in the number of daily steps occurred between pre-operative baseline and one week post-discharge, with corresponding worsening of symptom and QOL scores. We observed a potential correlation between fewer daily steps and a higher CCI score, indicating that patients with higher complications are less mobile, a trend that has been previously reported.42 Psychological and physical complaints may indeed limit a patient’s willingness to walk. By monitoring these symptoms closely, the surgical team and hospital staff has the opportunity to address them as early as possible and thus influence recovery time and overall QOL. The feedback system revealed that 33% of encounters generated alerts and thus created an opportunity for assessment and communication with patients as most of the alerts were related to symptoms.
An interesting finding from the present study is the discordance between objective and subjective functional recovery. At 2 weeks post-operatively, patients report a return to baseline in terms of symptoms and QOL; however their mobility and number of steps lags behind. While patients may not report complaints during their post-operative visit, they might benefit from encouragement or more aggressive measures such as a consultation with a physiotherapist.
Study findings suggest that our wireless program incorporating both subjective and objective parameters of recovery have the potential to provide opportunities for tailored post-operative care. For example, patients discharged home prior to day 7 with a poor functional status could be seen in clinic earlier or can undergo a physician telephonic evaluation, in an attempt to detect complications earlier and thus prevent readmissions.
There are several limitations to this study that warrant further discussion. First, an important limitation of this study is the small sample size. This was derived to be realistic and practical for the short study timeframe, and our intent was to determine, as a proof of concept whether the current wireless monitoring program can be administered prospectively and continuously in the peri-operative setting. Second, our study population included a variety of operation types with different risk profiles, which can impact study findings. Although a more homogeneous population (i.e. focused on liver or gastric surgery patients only) could make it easier for certain comparisons, the present population of diverse abdominal surgeries for various GI malignancies reflects the ultimate population in which our program could be implemented despite their various complication profiles and post-operative course. A third limitation is the nature of the patient population, which was of higher socio-economic status, well educated and technologically savvy. The adherence and feasibility of this program might be different in a more diverse population. To address these limitations, we are planning on conducting a broader 283 patient study in geographically and socio-economically diverse English and Spanish speaking populations. The large trial will also provide the opportunity to examine potential differences in functional recovery based on type of operation and risk profiles.
Important strengths of this study include the assessment of pre-operative data that served as a baseline and the use of both subjective and objective measures of patient centered functional recovery. Another strength is the portability and adaptability of the developed monitoring program to other clinical settings. Moreover, given the wireless nature of the technology, monitoring could be performed in a centralized fashion. Further studies on larger populations need to be performed to confirm associations between outcomes as well as to devise real-time interventions based on the data collected. Moreover a longer period of monitoring up to 60 days might be beneficial in order to truly capture patient return to baseline.
Conclusions
We conclude that wireless monitoring of patient-centered outcomes can be carried out both before and after major abdominal cancer surgery. A future large, prospective, longitudinal trial will determine whether these outcomes can augment existing surgical prediction tools and provide a more patient-centered approach to measuring quality in surgical oncology. Wireless technology has potential to detect real-time changes in symptom severity, mobility, and QOL and could provide opportunities for early intervention, when deviations from the expected post-operative course occur.
Key Points.
Question: Can wireless monitoring of patient –centered outcomes and recovery be carried out before and after major abdominal cancer surgery?
Findings: In this pilot study that included 20 patients with 160 monitoring encounters, functional recovery monitoring using wristband pedometers can be carried out with up to 88% adherence.
Meaning: Wireless monitoring of functional recovery and patient-reported outcomes has the potential for early interventions by transforming data into actionable patient care.
Acknowledgments
The authors have no disclosures to report. The research reported in this paper was supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572. It included work performed in the Survey Research Core and Biostatistics Core supported under the award number. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or NIH.
Dr. Sun had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors thank Indra Mahajan for her precious help in the editing and preparation of the manuscript.
Footnotes
Meeting Presentation: Scientific Forum, American College of Surgeons Clinical Congress, Washington, DC, October 17, 2016.
Trial Registration: NCT02511821
References
- 1.Pugalenthi A, Protic M, Gonen M, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. Journal of surgical oncology. 2016 Feb;113(2):188–193. doi: 10.1002/jso.24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heerkens HD, Tseng DS, Lips IM, et al. Health-related quality of life after pancreatic resection for malignancy. The British journal of surgery. 2016 Feb;103(3):257–266. doi: 10.1002/bjs.10032. [DOI] [PubMed] [Google Scholar]
- 3.Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Annals of surgery. 2015 Feb;261(2):345–352. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 4.Maillard J, Elia N, Haller CS, Delhumeau C, Walder B. Preoperative and early postoperative quality of life after major surgery - a prospective observational study. Health and quality of life outcomes. 2015 Feb 4;13(1):12. doi: 10.1186/s12955-014-0194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isik O, Okkabaz N, Hammel J, Remzi FH, Gorgun E. Preoperative functional health status may predict outcomes after elective colorectal surgery for malignancy. Surgical endoscopy. 2015 May;29(5):1051–1056. doi: 10.1007/s00464-014-3777-2. [DOI] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? Journal of the American College of Surgeons. 2004 Apr;198(4):626–632. doi: 10.1016/j.jamcollsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: The National Academies; 2013. [PubMed] [Google Scholar]
- 8.Chung AE, Basch EM. Potential and Challenges of Patient-Generated Health Data for High-Quality Cancer Care. J Oncol Pract. 2015 Apr 7; doi: 10.1200/JOP.2015.003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbach DR, Harnish JL, Long G. Short-term health-related quality of life after abdominal surgery: a conceptual framework. Surgical innovation. 2005 Sep;12(3):243–247. doi: 10.1177/155335060501200310. [DOI] [PubMed] [Google Scholar]
- 10.Swanson RS, Pezzi CM, Mallin K, Loomis AM, Winchester DP. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the national cancer data base. Annals of surgical oncology. 2014 Dec;21(13):4059–4067. doi: 10.1245/s10434-014-4036-4. [DOI] [PubMed] [Google Scholar]
- 11.Wagner D, Buttner S, Kim Y, et al. Clinical and morphometric parameters of frailty for prediction of mortality following hepatopancreaticobiliary surgery in the elderly. The British journal of surgery. 2016 Jan;103(2):e83–92. doi: 10.1002/bjs.10037. [DOI] [PubMed] [Google Scholar]
- 12.Reddy S, Contreras CM, Singletary B, et al. Timed Stair Climbing Is the Single Strongest Predictor of Perioperative Complications in Patients Undergoing Abdominal Surgery. Journal of the American College of Surgeons. 2016 Apr;222(4):559–566. doi: 10.1016/j.jamcollsurg.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook DJ, Thompson JE, Prinsen SK, Dearani JA, Deschamps C. Functional recovery in the elderly after major surgery: assessment of mobility recovery using wireless technology. The Annals of thoracic surgery. 2013 Sep;96(3):1057–1061. doi: 10.1016/j.athoracsur.2013.05.092. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. Journal of the American College of Surgeons. 2004 Nov;199(5):762–772. doi: 10.1016/j.jamcollsurg.2004.05.280. [DOI] [PubMed] [Google Scholar]
- 15.Ferriolli E, Skipworth RJ, Hendry P, et al. Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? Journal of pain and symptom management. 2012 Jun;43(6):1025–1035. doi: 10.1016/j.jpainsymman.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Huebner M, Hubner M, Cima RR, Larson DW. Timing of complications and length of stay after rectal cancer surgery. Journal of the American College of Surgeons. 2014 May;218(5):914–919. doi: 10.1016/j.jamcollsurg.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 17.Leidy NK. Functional status and the forward progress of merry-go-rounds: toward a coherent analytical framework. Nursing research. 1994 Jul-Aug;43(4):196–202. [PubMed] [Google Scholar]
- 18.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. Jama. 1995 Jan 4;273(1):59–65. [PubMed] [Google Scholar]
- 19.Rees JR, Blazeby JM, Fayers P, et al. Patient-reported outcomes after hepatic resection of colorectal cancer metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Apr 20;30(12):1364–1370. doi: 10.1200/JCO.2011.38.6177. [DOI] [PubMed] [Google Scholar]
- 20.Bennett AV, Reeve BB, Basch EM, et al. Evaluation of pedometry as a patient-centered outcome in patients undergoing hematopoietic cell transplant (HCT): a comparison of pedometry and patient reports of symptoms, health, and quality of life. Qual Life Res. 2015 Nov 17;:1–12. doi: 10.1007/s11136-015-1179-0. [DOI] [PubMed] [Google Scholar]
- 21.Kooiman TJ, Dontje ML, Sprenger SR, Krijnen WP, van der Schans CP, de Groot M. Reliability and validity of ten consumer activity trackers. BMC sports science, medicine and rehabilitation. 2015;7(1):24. doi: 10.1186/s13102-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000 Oct 1;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012 Jul-Aug;15(5):708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Luo N, Johnson JA, Shaw JW, Coons SJ. Relative efficiency of the EQ-5D, HUI2, and HUI3 index scores in measuring health burden of chronic medical conditions in a population health survey in the United States. Medical care. 2009 Jan;47(1):53–60. doi: 10.1097/MLR.0b013e31817d92f8. [DOI] [PubMed] [Google Scholar]
- 25.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Medical care. 2005 Mar;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Medical care. 2005 Nov;43(11):1078–1086. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 27.Slankamenac K, Nederlof N, Pessaux P, et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Annals of surgery. 2014 Nov;260(5):757–762. doi: 10.1097/SLA.0000000000000948. discussion 762–753. [DOI] [PubMed] [Google Scholar]
- 28.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Annals of surgery. 2013 Jul;258(1):1–7. doi: 10.1097/SLA.0b013e318296c732. [DOI] [PubMed] [Google Scholar]
- 29.Jensen RE, Snyder CF, Abernethy AP, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. Journal of oncology practice/American Society of Clinical Oncology. 2014 Jul;10(4):e215–222. doi: 10.1200/JOP.2013.001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basch E, Artz D, Dulko D, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 May 20;23(15):3552–3561. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 31.Taenzer P, Bultz BD, Carlson LE, et al. Impact of computerized quality of life screening on physician behaviour and patient satisfaction in lung cancer outpatients. Psychooncology. 2000 May-Jun;9(3):203–213. doi: 10.1002/1099-1611(200005/06)9:3<203::aid-pon453>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 32.Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psychooncology. 2007 Dec;16(12):1069–1079. doi: 10.1002/pon.1184. [DOI] [PubMed] [Google Scholar]
- 33.Boyes A, Newell S, Girgis A, McElduff P, Sanson-Fisher R. Does routine assessment and real-time feedback improve cancer patients’ psychosocial well-being? Eur J Cancer Care (Engl) 2006 May;15(2):163–171. doi: 10.1111/j.1365-2354.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 34.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004 Feb 15;22(4):714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 35.Mooney KH, Beck SL, Friedman RH, Farzanfar R. Telephone-linked care for cancer symptom monitoring: a pilot study. Cancer Pract. 2002 May-Jun;10(3):147–154. doi: 10.1046/j.1523-5394.2002.103006.x. [DOI] [PubMed] [Google Scholar]
- 36.Cleeland CS, Wang XS, Shi Q, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Mar 10;29(8):994–1000. doi: 10.1200/JCO.2010.29.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weaver A, Young AM, Rowntree J, et al. Application of mobile phone technology for managing chemotherapy-associated side-effects. Ann Oncol. 2007 Nov;18(11):1887–1892. doi: 10.1093/annonc/mdm354. [DOI] [PubMed] [Google Scholar]
- 38.Butt Z, Wagner LI, Beaumont JL, et al. Longitudinal screening and management of fatigue, pain, and emotional distress associated with cancer therapy. Support Care Cancer. 2008 Feb;16(2):151–159. doi: 10.1007/s00520-007-0291-2. [DOI] [PubMed] [Google Scholar]
- 39.Kearney N, McCann L, Norrie J, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer. 2009 Apr;17(4):437–444. doi: 10.1007/s00520-008-0515-0. [DOI] [PubMed] [Google Scholar]
- 40.Bainbridge D, Seow H, Sussman J, et al. Multidisciplinary health care professionals’ perceptions of the use and utility of a symptom assessment system for oncology patients. J Oncol Pract. 2011 Jan;7(1):19–23. doi: 10.1200/JOP.2010.000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basch E, Snyder C, McNiff K, et al. Patient-reported outcome performance measures in oncology. J Oncol Pract. 2014 May;10(3):209–211. doi: 10.1200/JOP.2014.001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA: a cancer journal for clinicians. 2012 Sep-Oct;62(5):337–347. doi: 10.3322/caac.21150. [DOI] [PubMed] [Google Scholar]
- 43.Strasser F, Blum D, von Moos R, et al. The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E-MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06) Ann Oncol. 2016 Feb;27(2):324–332. doi: 10.1093/annonc/mdv576. [DOI] [PubMed] [Google Scholar]
- 44.Nekhlyudov L, Levit L, Hurria A, Ganz PA. Patient-centered, evidence-based, and cost-conscious cancer care across the continuum: Translating the Institute of Medicine report into clinical practice. CA Cancer J Clin. 2014 Nov-Dec;64(6):408–421. doi: 10.3322/caac.21249. [DOI] [PubMed] [Google Scholar]
- 45.Dawes AJ, Reardon S, Chen VL, et al. Wireless Technology to Track Surgical Patients after Discharge: A Pilot Study. Am Surg. 2015 Oct;81(10):1061–1066. [PubMed] [Google Scholar]


