Figure 1. Design variables to be considered when developing biomaterial applications for myocardial infarction (MI) and peripheral artery disease (PAD).
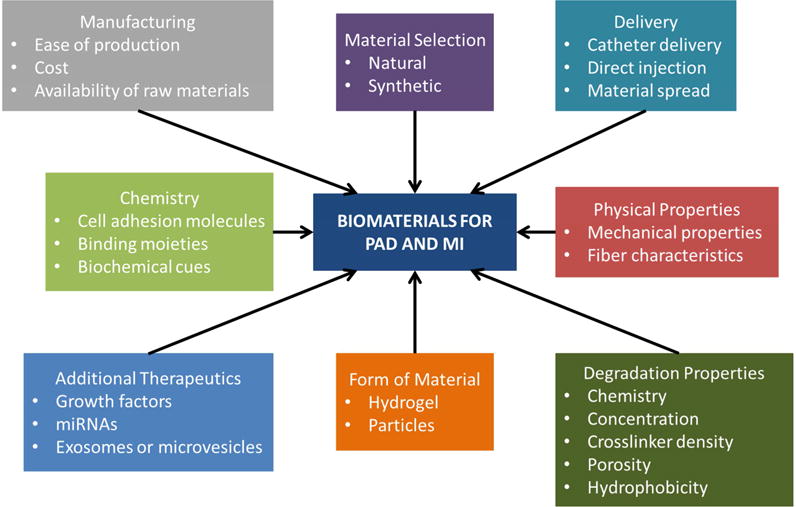
To successfully translate biomaterials to the clinic, specific design criteria must be considered to ensure the final product remains biocompatible while maintaining its full therapeutic efficacy. Extensive engineering of a biomaterial can maximize therapeutic benefits, but these benefits must counterbalance accompanied costs and manufacturing difficulties.
