Abstract
IMPORTANCE
Perinatal transmission of human immunodeficiency virus (HIV) can be reduced through services including antiretroviral treatment and prophylaxis. Data on the national incidence of perinatal HIV transmission and missed prevention opportunities are needed to monitor progress toward elimination of mother-to-child HIV transmission.
OBJECTIVE
To estimate the number of perinatal HIV cases among infants born in the United States.
DESIGN, SETTING, AND PARTICIPANTS
Data were obtained from the National HIV Surveillance System on infants with HIV born in the United States (including the District of Columbia) and their mothers between 2002 and 2013 (reported through December 31, 2015). Estimates were adjusted for delay in diagnosis and reporting by weighting each reported case based on a model incorporating time from birth to diagnosis and report. Analysis was performed from April 1 to August 15, 2016.
EXPOSURES
Maternal HIV infection and antiretroviral medication, including maternal receipt prenatally or during labor/delivery and infant receipt postnatally.
MAIN OUTCOMES AND MEASURES
Diagnosis of perinatally acquired HIV infection in infants born in the United States. Infant and maternal characteristics, including receipt of perinatal HIV testing, treatment, and prophylaxis.
RESULTS
The estimated annual number of perinatally infected infants born in the United States decreased from 216 (95% CI, 206–230) in 2002 to 69 (95% CI, 60–83) in 2013. Among perinatally HIV-infected children born in 2002–2013, 836 (63.0%) of the mothers identified as black or African American and 243 (18.3%) as Hispanic or Latino. A total of 236 (37.5%) of the mothers had HIV infection diagnosed before pregnancy in 2002–2005 compared with 120 (51.5%) in 2010–2013; the proportion of mother-infant pairs receiving all 3 recommended arms of antiretroviral prophylaxis or treatment (prenatal, intrapartum, and postnatal) was 22.4% in 2002–2005 and 31.8% in 2010–2013, with approximately 179 (28.4%) (2002–2005) and 94 (40.3%) (2010–2013) receiving antiretroviral prophylaxis or treatment during pregnancy. Five Southern states (Florida, Texas, Georgia, Louisiana, and Maryland) accounted for 687 (38.0%) of infants born with HIV infection in the United States during the overall period. According to national data for live births, the incidence of perinatal HIV infection among infants born in the United States in 2013 was 1.75 per 100 000 live births.
CONCLUSIONS AND RELEVANCE
Despite reduced perinatal HIV infection in the United States, missed opportunities for prevention were common among infected infants and their mothers in recent years. As of 2013, the incidence of perinatal HIV infection remained 1.75 times the proposed Centers for Disease Control and Prevention elimination of mother-to-child HIV transmission goal of 1 per 100 000 live births.
Highly effective interventions to prevent perinatal transmission of human immunodeficiency virus (HIV) have been available for a number of years. Beginning in 1985, the Centers for Disease Control and Prevention (CDC) recommended that HIV-infected women not breastfeed their infants1; routine administration of antiretroviral (ARV) medication for prophylaxis, zidovudine, of HIV-infected pregnant women,2 and the offer of prenatal HIV testing to all pregnant women3 were recommended in 1994 and 1995, respectively. The percentage of HIV-infected pregnant women receiving ARV prophylaxis rapidly increased after 1995.4,5 In 2006, the CDC recommended universal HIV testing—using an opt-out approach—for all pregnant women to increase screening in health care settings and further reduce perinatal HIV transmission in the United States.6
Suppression of maternal HIV viral load by potent therapeutic combinations became possible later in the 1990s with the availability of new categories of ARVs, such as protease inhibitors and nonnucleoside reverse transcriptase inhibitors. The use of these drugs in pregnant women in a manner similar to their use in nonpregnant women became accepted practice.7 It was also recognized that cesarean delivery before the onset of labor and rupture of membranes could further decrease perinatal HIV transmission risk, independent of ARV use8,9; the practice was recommended for women whose viral load was not known to be below 1000 copies/mL near the time of delivery.10 With optimal management, including suppression of HIV viral load to undetectable levels, mother-to-child HIV transmission rates lower than 1% can be achieved.11–13
Although identification of HIV infection is a necessary first step in appropriate treatment and prophylaxis, reports indicate that a quarter of pregnant women have not been tested.14,15 In addition, substantial proportions of women with known HIV infection did not receive adequate prenatal care or ARV medications.16
National estimates of the number of perinatal HIV transmissions in the United States are needed to guide policy and monitor progress toward elimination of mother-to-child transmission. Previous estimates of the number of perinatal HIV cases in the United States demonstrated a peak of 1650 in 1991, declining to 480 in 1996.5 Confidential name-based reporting of persons with AIDS began in all states and the District of Columbia in the early 1980s. In contrast, confidential name-based reporting of all persons with HIV infection (regardless of the stage of the disease at diagnosis) was implemented by states at different times, with 33 states having implemented name-based HIV reporting by January 2001, 38 states by January 2006, and 50 states and the District of Columbia by April 2008.17 Thus, the most recently published estimate of 138 (95% CI, 96–186) cases of perinatal HIV transmission for 200418 applied an indirect method using existing HIV and AIDS surveillance data to extrapolate from the 33 states with name-based HIV reporting data to all 50 US states, the District of Columbia, and 5 US-dependent areas. Our objective in the present study was to use existing HIV surveillance data to describe the characteristics of perinatally infected children born in 2002–2013 and known missed prevention opportunities, update the previous estimate to include birth years 2002–2013, and provide an estimate that reflects HIV reporting data from all 50 states and the District of Columbia in recent years.
Methods
Data Sets
Diagnosis data on HIV from 50 states and the District of Columbia reported to the National HIV Surveillance System of the CDC by December 31, 2015, were used to examine variables related to perinatal HIV transmission events from 2002 through 2013 in the United States and estimate the number of infants with HIV infection born in each of these years. Data reported through the National HIV Surveillance System are reported in accordance with state notifiable disease reporting laws and requirements. These data are nonresearch and do not require a waiver of informed consent.
Part 1: Characteristics of Perinatally HIV-Infected Infants Born in the United States, 2002–2013
We examined the characteristics of infants with perinatal HIV infection who were born in the United States between January 1, 2002, and December 31, 2013, and reported to the National HIV Surveillance System through December 31, 2015. Data were collected by state and local surveillance staff on a standardized Pediatric HIV Confidential Case Report form, and deidentified data were reported monthly to the CDC. Variables examined are listed in Table 1 in 4-year intervals: maternal race/ethnicity, number of maternal prenatal care visits, timing of prenatal care, timing of maternal HIV diagnosis, maternal receipt of any ARV therapy during pregnancy, maternal receipt of any ARV during labor and delivery, child’s receipt of any ARV, maternal and child receipt of any ARV agent at 3 time points (prenatal, labor and delivery, and postnatal), breastfeeding, and mode of delivery. Data for this section were not weighted to account for delays in diagnosis or reporting; therefore, the case counts for each year are lower than those in part 2, as described below (Table 2).
Table 1.
Selected Characteristics of US-Born Perinatally HIV-Infected Children and Their Mothersa
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| 2002–2005 | 2006–2009 | 2010–2013 | Total | |
| No. of cases | 630 | 464 | 233 | 1327 |
| Mother’s race/ethnicity | ||||
| Black or African American | 407 (64.6) | 280 (60.3) | 149 (63.9) | 836 (63.0) |
| Hispanic or Latinob | 120 (19.0) | 81 (17.5) | 42 (18.0) | 243 (18.3) |
| White | 58 (9.2) | 53 (11.4) | 19 (8.2) | 130 (9.8) |
| Other | 45 (7.1) | 50 (10.8) | 23 (9.9) | 118 (8.9) |
| Maternal prenatal care visits | ||||
| 0 | 61 (9.7) | 48 (10.3) | 39 (16.7) | 148 (11.2) |
| 1–10 | 186 (29.5) | 127 (27.4) | 74 (31.8) | 387 (29.2) |
| >10 | 56 (8.9) | 51 (11.0) | 36 (15.5) | 143 (10.8) |
| Missing | 327 (51.9) | 238 (51.3) | 84 (36.1) | 649 (48.9) |
| Start of prenatal care, month of pregnancy | ||||
| <7 | 225 (35.7) | 181 (39.0) | 119 (51.1) | 525 (39.6) |
| 7–9 | 48 (7.6) | 33 (7.1) | 16 (6.9) | 97 (7.3) |
| Other/unknown | 357 (56.7) | 250 (53.9) | 98 (42.1) | 705 (53.1) |
| Timing of mother’s HIV diagnosis | ||||
| Before pregnancy | 236 (37.5) | 199 (42.9) | 120 (51.5) | 555 (41.8) |
| During pregnancy | 108 (17.1) | 83 (17.9) | 41 (17.6) | 232 (17.5) |
| Before delivery | 44 (7.0) | 44 (9.5) | 18 (7.7) | 106 (8.0) |
| At delivery | 17 (2.7) | 10 (2.2) | 6 (2.6) | 33 (2.5) |
| After delivery | 174 (27.6) | 92 (19.8) | 43 (18.5) | 309 (23.3) |
| Timing unknown | 51 (8.1) | 36 (7.8) | 5 (2.1) | 92 (6.9) |
| Mother received any ARV during pregnancy | ||||
| Yes | 179 (28.4) | 157 (33.8) | 94 (40.3) | 430 (32.4) |
| No | 275 (43.7) | 165 (35.6) | 79 (33.9) | 519 (39.1) |
| Unknown | 176 (27.9) | 142 (30.6) | 60 (25.8) | 378 (28.5) |
| Mother received any ARV during L&D | ||||
| Yes | 214 (34.0) | 184 (39.7) | 116 (49.8) | 514 (38.7) |
| No | 244 (38.7) | 134 (28.9) | 51 (21.9) | 429 (32.3) |
| Unknown | 172 (27.3) | 146 (31.5) | 66 (28.3) | 384 (28.9) |
| Child received anyARV | ||||
| Yes | 362 (57.5) | 291 (62.7) | 158 (67.8) | 811 (61.1) |
| No/unknown | 268 (42.5) | 173 (37.3) | 75 (32.2) | 516 (38.9) |
| Mother/child received ARV at 3 time points: prenatal, L&D, and postnatal | ||||
| Yes | 141 (22.4) | 122 (26.3) | 74 (31.8) | 337 (25.4) |
| No/unknown | 489 (77.6) | 342 (73.7) | 159 (68.2) | 990 (74.6) |
| Breastfeeding | ||||
| Yes | 75 (11.9) | 41 (8.8) | 24 (10.3) | 140 (10.6) |
| No | 398 (63.2) | 289 (62.3) | 150 (64.4) | 837 (63.1) |
| Unknown | 157 (24.9) | 134 (28.9) | 59 (25.3) | 350 (26.4) |
| Mode of delivery | ||||
| Vaginal | 276 (43.8) | 175 (37.7) | 76 (32.6) | 527 (39.7) |
| Elective cesarean | 178 (28.3) | 152 (32.8) | 92 (39.5) | 422 (31.8) |
| Nonelective cesarean | 51 (8.1) | 43 (9.3) | 35 (15.0) | 129 (9.7) |
| Other/unknown | 125 (19.8) | 94 (20.3) | 30 (12.9) | 249 (18.8) |
Abbreviations: ARV, antiretroviral; HIV, human immunodeficiency virus; L&D, labor and delivery.
Infants with perinatal HIV infection who were born in the United States between January 2002 and December 2013 and reported to the National HIV Surveillance System through December 2015. Data were not weighted to account for delays in diagnosis or reporting; therefore, the case counts are lower than those in Table 2.
Hispanic and Latino can be of any race.
Table 2.
Estimated US-Born Perinatally HIV-Infected Infantsa
| Birth Year | No. (%) | Total (95% CI)c | |||
|---|---|---|---|---|---|
| Black/African American | Hispanic/Latinob | White | All Others | ||
| 2002 | 123 (56.9) | 41 (19.0) | 30 (13.9) | 22 (10.2) | 216 (206–230) |
| 2003 | 121 (64.0) | 40 (21.2) | 19 (10.1) | 9 (4.8) | 189 (180–202) |
| 2004 | 131 (64.5) | 38 (18.7) | 16 (7.9) | 18 (8.9) | 203 (193–216) |
| 2005 | 126 (69.2) | 31 (17.0) | 17 (9.3) | 8 (4.4) | 182 (172–196) |
| 2006 | 101 (57.7) | 24 (13.7) | 24 (13.7) | 26 (14.9) | 175 (164–190) |
| 2007 | 118 (62.1) | 34 (17.9) | 24 (12.6) | 14 (7.4) | 190 (177–207) |
| 2008 | 79 (51.6) | 23 (15.0) | 31 (20.3) | 19 (12.4) | 153 (141–170) |
| 2009 | 89 (65.9) | 30 (22.2) | 7 (5.2) | 9 (6.7) | 135 (124–149) |
| 2010 | 54 (51.4) | 23 (21.9) | 19 (18.1) | 9 (8.6) | 105 (95–120) |
| 2011 | 55 (61.1) | 18 (20.0) | 7 (7.8) | 9 (10.0) | 90 (81–104) |
| 2012 | 75 (72.8) | 13 (12.6) | 5 (4.9) | 11 (10.7) | 103 (92–118) |
| 2013 | 41 (59.4) | 10 (14.5) | 12 (17.4) | 6 (8.7) | 69 (60–83) |
Abbreviation: HIV, human immunodeficiency virus.
Data include persons with a diagnosis of HIV infection regardless of the stage of the disease at diagnosis. Estimated numbers resulted from statistical adjustment that accounted for delays between birth and diagnosis as well as diagnosis and reporting. The estimated annual percent change for the total count is −8.4% (95% CI, −9.7% to −7.2%; P < .001).
Hispanic and Latino can be of any race.
Estimated totals may not equal the sum of values in each row due to rounding.
Part 2: Estimated Number of Perinatally HIV-Infected Infants Born in the United States, 2002–2013
Estimates for the annual number of perinatal HIV infections that occurred in the entire United States from 2002 to 2013 were developed by adjusting the reported number of perinatal HIV cases to account for delay in diagnosis and reporting. A perinatally HIV-infected infant may not be diagnosed at birth, and a diagnosed case may not be reported to the system immediately. Therefore, the delay associated with the diagnosis and reporting of a perinatally HIV-infected infant is defined as the time from the date when a perinatally HIV-infected infant was born to the date when HIV infection was diagnosed and reported to the National HIV Surveillance System. Based on the clear biphasic delay pattern observed among reported cases, we estimated the probabilities that perinatally infected persons were diagnosed and reported within certain times using conditional probabilities. For delays longer than 4 years, we modeled the probability by a truncated exponential distribution. For delays less than 4 years, we modeled the probability by a nonparametric approach using the life-table method.19,20 Through this procedure, each reported case was assigned a weight to account not only for reported infants but also for infants born in each year who had not yet been reported to the CDC. The weight is the inverse of the diagnosis and reporting delay probability associated with the case. To determine whether there was a significant increasing or decreasing trend in the estimated annual numbers of perinatally HIV-infected infants, the estimated annual percent change and associated 95% CIs were calculated. A change in trend was considered statistically significant if the P value is <.05.21
Markov Chain Monte Carlo Simulation for 95% CI
Given the national estimate of perinatally infected infants in all 50 states and the District of Columbia, N = n×w, where N is the national estimate, n is the reported number of perinatal HIV cases by birth year, and w is the final reporting delay weight, we estimated the uncertainty around each of the 2 variables (n and w) and then used Markov Chain Monte Carlo simulation to calculate the 95% CI of the estimated number of perinatal HIV infections in each birth year. The uncertainty associated with each input was incorporated into the final estimate by randomly resampling each variable under a plausible probability distribution. In particular, the Poisson distribution was used for resampling the count data (n: yearly births of perinatal HIV and AIDS cases), and the binomial distribution was used for resampling the reporting probability (w = 1/reporting probability). Simulation results from 1000 runs for each estimate were used to compute the confidence intervals. Random sampling was applied in the Markov Chain Monte Carlo simulation procedure.22 The distribution of the values for each output estimate reflects the overall uncertainty that is associated with the input values. The 95% CIs were derived by identification of the 2.5 and 97.5 percentile values for the distribution of computational results. Using the estimated number of perinatal HIV infections, as described above, we then calculated the incidence of perinatal HIV infection as the estimated number of perinatal infections per 100 000 live births, with denominators based on national birth registry data.23 All analyses were performed by using SAS, version 9.3 (SAS Institute Inc). Analysis was performed from April 1 to August 15, 2016.
Results
Characteristics of Perinatally HIV-infected Infants
Nearly two-thirds (63.0%) of the mothers of perinatally HIV-infected infants born in the United States during this time period were black or African American, and 18.3% were Hispanic or Latino (Table 1). Nearly half (48.9%) of the mothers had missing or an unknown number of prenatal care visits, while 40.0% had at least 1 prenatal visit during the index pregnancy, and 11.2% had no reported prenatal care visits. The percentage of women having no reported prenatal care visits increased from 9.7% in 2002–2005 to 16.7% in 2010–2013. Prenatal care was started in the first or second trimester in 39.6% of pregnancies and during the third trimester in 7.3% (53.1% unknown).
Among the mothers of all perinatally HIV-infected infants born in 2002–2013, 41.8% had HIV infection diagnosed prior to the index pregnancy, with this proportion being 37.5% for mothers of children born during 2002–2005 and 51.5% for those with children born during 2010–2013. Diagnosis was made at or after delivery in 30.3% of the mothers during 2002–2005, compared with 21.6% during 2010–2013. Prenatal ARV medications were used by 179 (28.4%) of the mothers during 2002–2005 and 94 (40.3%) during 2010–2013. Overall, 25.4% of mother-infant pairs received ARV medications at all of the 3 recommended time points (ie, prenatally, during labor, and to the infant after birth) from 2002 through 2013, with this proportion being 22.4% during 2002–2005 and 31.8% during 2010–2013. Breastfeeding was reported by mothers of 10.6% of HIV-infected infants during 2002–2013 (range, 8.8%–11.9%). Bivariate analysis revealed that, between 2010 and 2013, 40.2% of women whose HIV was diagnosed before delivery received ARV medications during the pregnancy, delivery, and postnatal periods, compared with 4.0% of those with HIV diagnosed at delivery or later. Similarly, for the same time period, 42.7% of women with at least 1 prenatal care visit received ARV medications at all 3 time points compared with 7.6% of women with no prenatal care visits.
Estimated Number of Perinatal HIV Infections
Applying the reporting delay weights to the number of cases reported for birth years 2002–2013, we estimated that 216 (95% CI, 206 to 230) perinatal HIV infections occurred among infants born in the United States in 2002 (5.37 per 100 000 live births) (Table 2 and Figure 1). In 2013, we estimated that there were 69 (95% CI, 60 to 83) perinatal HIV infections (1.75 per 100 000 live births). The estimated annual percentage change from 2002 to 2013 was −8.4% (95% CI, −9.7% to −7.2%; P < .001). During the entire observation period (2002–2013), there were an estimated 1810 infants born with HIV infection in the United States, 1221 (67.5%) of whom were born in 10 states (in descending order: Florida, Texas, New York, Georgia, Pennsylvania, California, Illinois, Louisiana, New Jersey, and Maryland). Five of these states are in the South (Florida, Texas, Georgia, Louisiana, and Maryland) and accounted for 687 (38.0%) of the cases.
Figure 1.
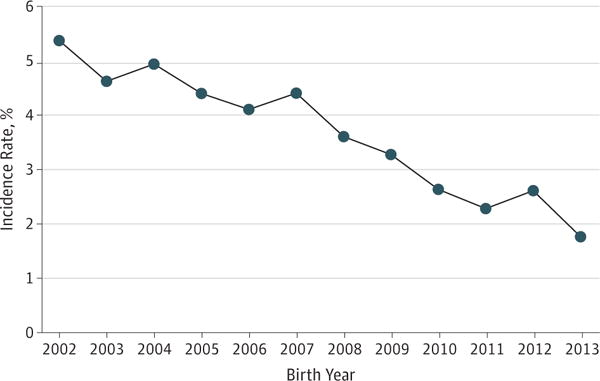
Estimated Incidence Rates of Perinatally Acquired Human Immunodeficiency Virus Infection in 50 US States and the District of Columbia, 2002–2013
Rates are estimated diagnoses per 100 000 live births and were adjusted for delay in reporting from birth to diagnosis and diagnosis to report.
During the most recent 4-year period (2010–2013), an estimated 134 infants were born in 3 states (in descending order: Florida, 48; Texas, 44; and Georgia, 42) constituting 36.2% of the estimated number of infants born. The denominator used for these calculations was based on the state data provided in Figure 2, which differ from the totals presented in Table 2 because of rounding. Eleven states had 10 or more infants born with HIV infection; HIV infection occurred in 275 infants in these 11 states, which accounted for 74.3% of the infants with HIV infection born during this period. The estimated incidence of perinatal HIV infection from 2010 to 2013 was less than 1.0 per 100 000 live births for 23 jurisdictions, 1.0 to 1.99 per 100 000 live births for 12 jurisdictions, 2.0 to 3.99 per 100 000 live births for 9 jurisdictions, and 4.0 or more per 100 000 live births for 7 jurisdictions (Figure 2). Among the 16 jurisdictions with an incidence of 2.0 or more per 100 000 live births, 10 were in the South.
Figure 2.
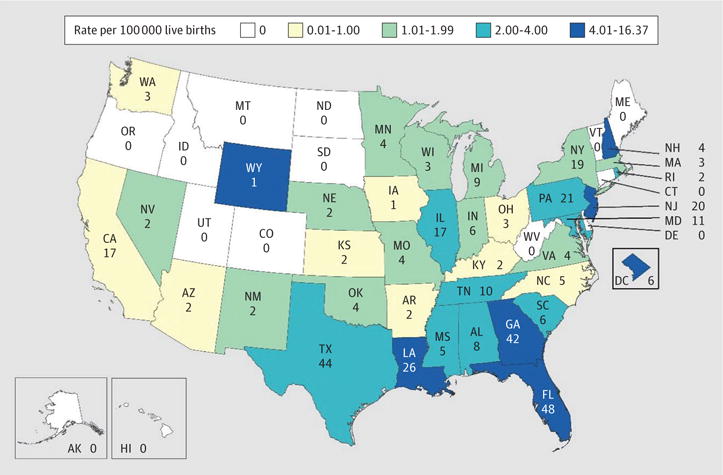
Estimated Numbers and Rates of Perinatally Acquired Human Immunodeficiency Virus Infections Among Children Born in the United States and the District of Columbia, 2010–2013
Estimated numbers less than 12 and rates based on them should be interpreted with caution because numbers less than 12 have underlying relative SEs greater than 30% and are considered unreliable. Inset maps not to scale.
Discussion
In this analysis, we examined characteristics of perinatally HIV-infected infants born in the United States between 2002 and 2013 based on national HIV surveillance data. Many mother-infant pairs had missed at least 1 prevention opportunity. The most common of these factors was lack of appropriate ARV medications during pregnancy; just 25.4% of HIV-infected mother-infant pairs were known to have received at least 1 ARV during all 3 recommended periods (pregnancy, delivery, and postnatal) over the 12-year period examined. Lack of ARV medications was associated with late maternal HIV diagnosis.
During the analysis period, we observed some positive changes in the percentage of HIV-infected infants and their mothers who had received recommended prevention interventions. Observed increases in ARV use remained lower than the goal of universal perinatal ARV prophylaxis for prevention of perinatal HIV transmission. The observed increase in the percentage of women with HIV diagnosed prior to pregnancy, which at face value seems contrary to the apparent increase in the percentage of women with no prenatal care during the latter years (2010–2013), may be indicative of increased HIV testing in general among women during this period.
The prevalence of missed opportunities observed in this study among all infants with diagnosed HIV born in the United States between 2002 and 2013 was consistent with that seen among HIV-infected women and their infants reported to the CDC’s Enhanced Perinatal HIV Surveillance System from 15 high-prevalence areas in the United States (2005–2008).16 Data from the Enhanced Perinatal HIV Surveillance System for 2005–2008 showed slight increases in the percentages of women with HIV diagnosed prior to pregnancy (from 66% to 71%), receiving prenatal ARV medications (from 82% to 87%), and receiving intrapartum ARV medications (from 81% to 88%), but they showed no apparent change in the percentage of pregnant women with prenatal care (90%).16
The continuing prevalence of missed prevention opportunities suggests that the remaining HIV transmissions occur in a subset of the population that is particularly difficult to reach with the recommended interventions.24 One could hypothesize that the difficulty in reaching this population may also be reflected by the increasing percentage of women who, although infection was diagnosed before pregnancy, had not apparently been successfully linked to—and retained in—care. Stated differently, the fact that infants continue to acquire HIV infection appears to be the result of the same reasons that have been known for years and likely not as a result of a previously unrecognized factor, such as increasing ARV resistance or an increasing incidence of women with acute HIV infection during pregnancy.
These findings suggest that new strategies and more intense public health interventions may be needed to maintain the achievements attained thus far and ultimately eliminate perinatal HIV transmission in the United States.25 Given the association that we observed between late maternal diagnosis and lack of ARV receipt, additional emphasis on preconception counseling of HIV-infected women could provide one opportunity to ensure that all women have an effectively suppressed viral load before delivery.26 Increased testing of partners of known HIV-infected adults can specify the type of preconception counseling that a couple needs. Many of the persons identified in this way will belong to HIV-discordant couples of reproductive age who could benefit from different approaches to planning safer conception and pregnancy, regardless of which partner is HIV infected.27 Acute HIV infection during pregnancy appears to be playing a consistent role in remaining transmissions,28–30 underscoring the usefulness of repeat HIV testing during pregnancy, as recommended by the CDC.6
The geographic distribution of these cases reflects the changing geography of HIV infections among women, with Southern states having a larger proportion of cases than in the past.5 The cases were concentrated in 10 states, highlighting the need to maintain great levels of capacity in these areas. However, the fact that cases were diagnosed in most jurisdictions during the overall study period highlights the need to maintain capacity in all states to detect and respond appropriately to perinatal HIV exposures and infections; it further suggests that an approach to prevention might include a detailed review of every case of transmission as a sentinel health event. It was for this reason that the CDC included in its 2012 HIV prevention grants to health departments support for a prevention strategy that includes detailed review of every transmission; this strategy is known as the Fetal-Infant Mortality Review–HIV Prevention Methodology.31 As of 2015, 9 jurisdictions had implemented this methodology.
Our estimates show important differences compared with the most recent prior published analysis of perinatal HIV trends in the United States, which estimated that 277 perinatal HIV infections had occurred in 2001, declining to 138 in 2004 and a total of 509 born during 2002–2004.18 Our analysis for 2002–2004 found approximately 20% more infections (n = 608) despite the fact that our estimate excluded infants born outside the United States. The prior estimates assumed that all cases would be reported to the system within 4 years.18 This assumption underestimated the number of perinatally HIV-infected infants during that study period. With more years’ data available for the present analysis and more jurisdictions reporting HIV diagnoses, we found that some cases were first reported up to 9 years after the birth year, and we modified our methodology to account for the longer observed reporting delays. The present study also updated these estimates for more recent years and found that the estimated number of perinatally acquired HIV infections in the United States subsequently declined from 182 in 2005 to 69 in 2013.
To our knowledge, this study provides the most geographically and temporally extensive data available describing substantial decreases in recent HIV transmissions to US-born infants and presents a national estimate of the incidence of perinatal HIV infection. In the most recent year available (2013), the incidence of perinatal HIV infection in the United States continued to be 1.75 times as high as the CDC perinatal HIV elimination incidence goal of 1 infection per 100 000 live births.25 The other component of that elimination goal is a mother-to-child transmission rate of less than 1% (the number of infected infants divided by the number of HIV-infected women delivering). There are no specific data on the number of HIV-exposed deliveries in the United States, and the degree to which the most recent estimate (approximately 8700 in 2006) correlates with the present number is unknown. Our analysis suggests that the elimination of mother-to-child transmission goal was not met in 2013. Although specific definitions of elimination vary, declaring such a goal is an implicit recognition that the feat is believed possible. Important in achieving that goal is public health involvement directed at further improvements in prenatal care utilization, identification of HIV-infected pregnant women (and retaining in care women whose HIV infection has been identified), and assurance of the delivery of appropriate interventions to maximize women’s and infants’ health. New HIV infections occurring in women, continuing large numbers of HIV-infected pregnant women delivering annually,32 and a higher incidence in infants of black or African American mothers continue despite declining perinatal HIV transmission overall.
Limitations
This analysis is subject to several important limitations. Some data were unavailable for the analysis of characteristics of diagnosed perinatal HIV infections, including maternal viral load, use of illicit substances by mothers of infected infants, mental health issues for the mother, and socioeconomic status. For the purposes of this analysis, a 3-part ARV regimen refers to the use of any ARV by the mother during the pregnancy and during labor and orally by the infant after delivery since the case report form does not distinguish triple-drug from single-drug regimens. Data for the timing of the maternal HIV diagnosis were collected in the categories reported in Table 1; precise dates of diagnosis and pregnancy were not consistently available. It was thus not possible to characterize more specifically the length of time between maternal diagnosis and birth of the infant. In addition, there was a substantial amount of missing data for some of the variables in Table 1. Although the national estimates of perinatal HIV infections were adjusted for diagnosis and reporting delays, descriptive data in Table 1 were not weighted because the weights were created in a model without considering those variables. Reported numbers less than 12, as well as estimated numbers and rates based on them, should be interpreted with caution because numbers have underlying relative SEs greater than 30% and are considered unreliable. Finally, data were available only for HIV-infected infants—not for all HIV-exposed infants. Data on HIV-exposed infants would allow more direct assessment of factors associated with transmission of HIV. Such data are no longer consistently available since the 2011 retirement of the Enhanced Perinatal HIV Surveillance System surveillance project.
Conclusions
Even if perinatal rates in the “elimination” range are achieved in any given year or jurisdiction, it will be necessary to sustain efforts to prevent perinatal HIV transmission. These efforts can include innovative approaches to reduce missed prevention opportunities and health disparities, as well as improve early testing, linkage to, and retention in care for women with HIV infection.
Key Points.
Question
What are the numbers and characteristics of infants with perinatal human immunodeficiency virus (HIV) infection born in the United States in recent years?
Findings
Data reported to the National HIV Surveillance System show that the estimated number of infants born with perinatal HIV infection decreased from 216 in 2002 to 69 in 2013. Maternal and infant factors associated with infant HIV infection include late maternal diagnosis and lack of antiretroviral treatment and prophylaxis.
Meaning
Despite reductions in perinatal HIV transmission in the United States, gaps in HIV diagnosis and treatment persist.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Contributions: Drs Zhang and Song had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Taylor, Nesheim, Zhang, Lampe, Weidle.
Acquisition, analysis, or interpretation of data: Taylor, Nesheim, Zhang, Song, FitzHarris, Weidle, Sweeney.
Drafting of the manuscript: Taylor, Nesheim, Zhang, Song, Weidle.
Critical revision of the manuscript for important intellectual content: Taylor, Nesheim, FitzHarris, Lampe, Weidle, Sweeney.
Statistical analysis: Taylor, Zhang, Song.
Administrative, technical, or material support: Nesheim, FitzHarris, Lampe, Sweeney. Supervision: Taylor, Nesheim, Weidle.
Additional Information: All of the authors are employees (or contractors, Ms FitzHarris) of the Centers for Disease Control and Prevention, which supported the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Allan W. Taylor, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, GeorgiaThe Center for Global Health, Office of the Director, Centers for Disease Control and Prevention, Atlanta, Georgia.
Steven R. Nesheim, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia.
Xinjian Zhang, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia.
Ruiguang Song, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia.
Lauren F. FitzHarris, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, GeorgiaICF International, Atlanta, Georgia.
Margaret A. Lampe, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia.
Paul J. Weidle, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia.
Patricia Sweeney, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia.
References
- 1.Centers for Disease Control (CDC) Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep. 1985;34(48):721–726. 731–732. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Recommendations of the US Public Health Service Task Force on the use of zidovudine to reduce perinatal transmission of human immunodeficiency virus. MMWR Recomm Rep. 1994;43(RR-11):1–20. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. US Public Health Service recommendations for human immunodeficiency virus counseling and voluntary testing for pregnant women. MMWR Recomm Rep. 1995;44(RR-7):1–15. [PubMed] [Google Scholar]
- 4.Simonds RJ, Steketee R, Nesheim S, et al. Perinatal AIDS Collaborative Transmission Studies Impact of zidovudine use on risk and risk factors for perinatal transmission of HIV. AIDS. 1998;12(3):301–308. doi: 10.1097/00002030-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Lindegren ML, Byers RH, Jr, Thomas P, et al. Trends in perinatal transmission of HIV/AIDS in the United States. JAMA. 1999;282(6):531–538. doi: 10.1001/jama.282.6.531. [DOI] [PubMed] [Google Scholar]
- 6.Branson BM, Handsfield HH, Lampe MA, et al. Centers for Disease Control and Prevention (CDC) Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Public Health Service Task Force recommendations for the use of antiretroviral drugs in pregnant women infected with HIV-1 for maternal health and for reducing perinatal HIV-1 transmission in the United States. MMWR Recomm Rep. 1998;47(RR-2):1–30. [PubMed] [Google Scholar]
- 8.European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353(9158):1035–1039. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 9.The International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340(13):977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Obstetric Practice. ACOG committee opinion scheduled Cesarean delivery and the prevention of vertical transmission of HIV infection; number 234, May 2000 (replaces number 219, August 1999) Int J Gynaecol Obstet. 2001;73(3):279–281. doi: 10.1016/s0020-7292(01)00412-x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper ER, Charurat M, Burns DN, Blattner W, Hoff R, The Women and Infants Transmission Study Group Trends in antiretroviral therapy and mother-infant transmission of HIV. J Acquir Immune Defic Syndr. 2000;24(1):45–47. doi: 10.1097/00126334-200005010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS. 2014;28(7):1049–1057. doi: 10.1097/QAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 13.Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209(4):335.e1–335.e12. doi: 10.1016/j.ajog.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JE, Sansom S. HIV testing among U.S. women during prenatal care: findings from the 2002 National Survey of Family Growth. Matern Child Health J. 2006;10(5):413–417. doi: 10.1007/s10995-006-0120-0. [DOI] [PubMed] [Google Scholar]
- 15.Fitz Harris LF, Taylor AW, Zhang F, et al. Factors associated with human immunodeficiency virus screening of women during pregnancy, labor and delivery, United States, 2005–2006. Matern Child Health J. 2014;18(3):648–656. doi: 10.1007/s10995-013-1289-7. [DOI] [PubMed] [Google Scholar]
- 16.Whitmore SK, Taylor AW, Espinoza L, Shouse RL, Lampe MA, Nesheim S. Correlates of mother-to-child transmission of HIV in the United States and Puerto Rico. Pediatrics. 2012;129(1):e74–e81. doi: 10.1542/peds.2010-3691. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. HIV Surveillance Report. 2010 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Updated January 3, 2017. Accessed May 3, 2012.
- 18.McKenna MT, Hu X. Recent trends in the incidence and morbidity that are associated with perinatal human immunodeficiency virus infection in the United States. Am J Obstet Gynecol. 2007;1973(suppl):S10–S16. doi: 10.1016/j.ajog.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Song R, Green T. An improved approach to accounting for reporting delay in case surveillance system. JP J Biostat. 2012;7(1):1–14. [Google Scholar]
- 20.Brookmeyer R, Liao JG. The analysis of delays in disease reporting: methods and results for the acquired immunodeficiency syndrome. Am J Epidemiol. 1990;132(2):355–365. doi: 10.1093/oxfordjournals.aje.a115665. [DOI] [PubMed] [Google Scholar]
- 21.Estève J, Benhamou E, Raymond L. Statistical methods in cancer research; volume IV: descriptive epidemiology. IARC Sci Publ. 1994;(128):1–302. [PubMed] [Google Scholar]
- 22.Marino S, Hogue IB, Ray CJ, Kirschner DE. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol. 2008;254(1):178–196. doi: 10.1016/j.jtbi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention; National Vital Statistics Report. Births final data for 2014. 64(12) https://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_12.pdf. Published December 23, 2015. Accessed February 16, 2017. [PubMed] [Google Scholar]
- 24.Lindau ST, Jerome J, Miller K, Monk E, Garcia P, Cohen M. Mothers on the margins: implications for eradicating perinatal HIV. Soc Sci Med. 2006;62(1):59–69. doi: 10.1016/j.socscimed.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Nesheim S, Taylor A, Lampe MA, et al. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics. 2012;130(4):738–744. doi: 10.1542/peds.2012-0194. [DOI] [PubMed] [Google Scholar]
- 26.Huntington SE, Bansi LK, Thorne C, et al. UK Collaborative HIV Cohort (UK CHIC) Study and the National Study of HIV in Pregnancy and Childhood (NSHPC). Treatment switches during pregnancy among HIV-positive women on antiretroviral therapy at conception. AIDS. 2011;25(13):1647–1655. doi: 10.1097/QAD.0b013e32834982af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol. 2011;204(6):4883.e1–488.e8. doi: 10.1016/j.ajog.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol. 2010;115(6):1247–1255. doi: 10.1097/AOG.0b013e3181e00955. [DOI] [PubMed] [Google Scholar]
- 29.Patterson KB, Leone PA, Fiscus SA, et al. Frequent detection of acute HIV infection in pregnant women. AIDS. 2007;21(17):2303–2308. doi: 10.1097/QAD.0b013e3282f155da. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Lampe MA, Surendura Babu A, Rao S, Borkowf CB, Nesheim SR. HIV Seroconversion During Pregnancy and Mother-to-Child HIV Transmission: Data From Enhanced Perinatal Surveillance, United States, 2005–2010. Washington, DC: International AIDS Society; 2012. [Google Scholar]
- 31.Department of Health and Human Services. CDC-RFA-PS12-1201. Comprehensive HIV Prevention Programs for Health Departments: catalog of Federal Domestic Assistance No.: 93.940. 2012 https://www.cdc.gov/hiv/pdf/funding/announcements/ps12-1201/cdc-hiv-ps12-1201.pdf. Accessed February 16, 2017.
- 32.Whitmore SK, Zhang X, Taylor AW, Blair JM. Estimated number of infants born to HIV-infected women in the United States and five dependent areas, 2006. J Acquir Immune Defic Syndr. 2011;57(3):218–222. doi: 10.1097/QAI.0b013e3182167dec. [DOI] [PubMed] [Google Scholar]


