Abstract
BACKGROUND AND PURPOSE
Posterior reversible encephalopathy syndrome is a clinicoradiologic syndrome. Literature regarding associated factors and the prognostic significance of contrast enhancement in posterior reversible encephalopathy syndrome is sparse. This study set out to evaluate an association between the presence of enhancement in posterior reversible encephalopathy syndrome and various clinical factors in a large series of patients with this syndrome.
MATERIALS AND METHODS
From an MR imaging report search that yielded 176 patients with clinically confirmed posterior reversible encephalopathy syndrome between 1997 and 2014, we identified 135 patients who had received gadolinium-based contrast. The presenting symptoms, etiology, clinical follow-up, and maximum systolic and diastolic blood pressures within 1 day of MR imaging were recorded. MRIs were reviewed for parenchymal hemorrhage, MR imaging severity, and the presence and pattern of contrast enhancement. Statistical analyses evaluated a correlation between any clinical features and the presence or pattern of enhancement.
RESULTS
Of 135 included patients (67.4% females; age range, 7–82 years), 59 (43.7%) had contrast enhancement on T1-weighted MR imaging, the most common pattern being leptomeningeal (n=24, 17.8%) or leptomeningeal plus cortical (n=21, 15.6%). Clinical outcomes were available in 96 patients. No significant association was found between the presence or pattern of enhancement and any of the variables, including sex, age, symptom, MR imaging severity, blood pressure, or outcome (all P >.05 after Bonferroni correction).
CONCLUSIONS
The presence or pattern of enhancement in posterior reversible encephalopathy syndrome is not associated with any of the tested variables. However, an association was found between MR imaging severity and clinical outcome.
Posterior reversible encephalopathy syndrome (PRES) is a clinical and radiographic syndrome that may result from various etiologies but is most commonly associated with hypertension, eclampsia, or treatment with immunosuppressant medications.1–4 The exact pathophysiology of this condition remains uncertain, but it has been postulated to relate to dysfunction in cerebral autoregulation or endothelial injury. Contrast enhancement can variably be present on MR imaging in patients with PRES, having been described in up to 38% of patients, but the significance of this is unclear. The presence of contrast enhancement implies dysfunction of the blood-brain barrier, and it has been postulated that the presence of enhancement in many patients with PRES may further point to endothelial injury as a possible cause.4–8
Whether the presence or pattern of contrast enhancement is related to disease severity, etiology, or prognosis is currently unknown because the umbrella of PRES encompasses a vast array of etiologies and imaging appearances. While the clinical picture of PRES is variable, not all cases are fully reversible.7,8 Typically, the use of gadolinium-based contrast is not necessary to solidify the diagnosis of PRES because the typical imaging appearance of cortical and/or subcortical edema on FLAIR or T2WI, with a corroborative clinical history, is decisive.1,7,9–13 Meanwhile, although most of the prior literature has described the edema patterns of PRES on FLAIR or T2WI, such studies have hardly focused on the patterns of enhancement or the clinical implications of such enhancement.7–12 Several smaller series have described the incidence and features of enhancement on MR imaging in PRES, with variable frequency.3,4,7,8,14,15 Given such variability, this study set out to review a larger series of patients with clinically confirmed PRES to determine whether an association could be made between the presence of contrast enhancement and etiology, prognosis, or a host of other clinical factors.
MATERIALS AND METHODS
Patient Selection
This retrospective study was approved by the institutional review boards of 2 hospitals, one being a tertiary care center and the other a level 1 trauma center. An MR imaging data base search was conducted for patients in whom the radiologic and clinical features of PRES were present within a 17-year period (1997–2014), yielding 176 patients with clinically confirmed PRES. The single inclusion criterion was having postcontrast T1WI in addition to the FLAIR images used to diagnose PRES. The exclusion criterion was either the lack of postcontrast T1WI or motion artifacts rendering the MR imaging uninterpretable. Patients with other comorbidities were not excluded, provided they were diagnosed with PRES.
The clinical criteria of PRES were defined as the presence of at least 1 acute neurologic deficit and clinical corroboration of suspected PRES on MR imaging. The radiologic features of PRES were defined by the presence of edema in characteristic distributions, as described previously.7,9,10 The medical records of these patients were also reviewed to retrieve patient demographics and clinical data, including sex, age, primary presenting symptom, presumed etiology, maximum systolic blood pressure (SBPmax) and maximum diastolic blood pressure (DBPmax) (both within 1 day of the MR imaging examination), and clinical outcome (if clinical follow-up was available). Regarding the clinical follow-up, the goal was a clinical examination performed at least 60 days after the initial presentation.
Clinical Severity and Outcome Scoring
Five categories of clinical outcome based on the follow-up evaluation were established on the basis of a prior study of PRES and acute toxic leukoencephalopathy, which consisted of the following: return to baseline clinical condition (grade 0); minimal residual cognitive deficit (grade 1); mild persistent neurologic deficit (grade 2); moderate persistent neurologic deficit (grade 3); and severe outcome including no improvement (eg, persistent), seizures, coma, or death (grade 4).16
MR Imaging Sequence Protocols
The MR imaging examinations were performed on 6 different scanners (4 with 1.5T magnet strength and 2 with 3T magnet strength) during the 17-year period, which routinely included axial T1WI, T2WI, T2 FLAIR images, DWI, and postcontrast T1WI.
Radiologic Severity and Grading
Retrospective review by consensus by 2 staff neuroradiologists (A.M.M., J.B.R.), with 12 and 4 years of experience respectively, was performed to evaluate the presence of intracranial hemorrhage, the pattern of radiologic severity, and the presence and pattern of contrast enhancement. Analysis of the clinical details of each case was performed separately, but with joint (consensus) grading of MR imaging severity. The grading of radiologic severity generally follows that previously used in the literature, with the addition of a new “minimal” grade (grade 1) to account for an earlier or milder form of PRES that has occurred with improving recognition of the disorder.4,7,9 This minimal grade was defined as symmetric, cortical involvement of only 1 lobe (frontal, temporal, parietal, or occipital) without involvement of the basal ganglia, brain stem, or deep white matter. The remaining degrees of “mild,” “moderate,” and “severe” grades adhere to the system previously described.7,9
When assessing the presence and pattern type of contrast enhancement, we further categorized the included patients as having leptomeningeal, cortical, or parenchymal nodular patterns of enhancement or a combination of any of these patterns, similar to that previously described.7
Statistical Analysis
An analysis of variance was performed to investigate whether sex, age, clinical presentation (symptom), DBPmax, SBPmax, MR imaging severity, or clinical outcome was associated with patterns of contrast enhancement and as an analysis to evaluate a correlation between the radiologic severity and clinical outcomes. P values were calculated from the 2-sample t test/1-way ANOVA for continuous variables and the Fisher exact test for categoric variables when comparing variables of interest by contrast enhancement. Thereafter, the mean and its 95% confidence intervals were calculated for continuous variables regarding the types of enhancement. A pair-wise comparison was performed after applying a Bonferroni adjustment for association with statistical significance, setting the P value at <.017 (P = .05/3). We applied a logistic regression to investigate predictors of contrast enhancement, calculating odds ratios and 95% confidence intervals. Notably, regarding outcome scoring, the grade 4 category (death) was merged with the “no follow-up” category to prevent the potential introduction of bias because most patients died from reasons other than PRES.
RESULTS
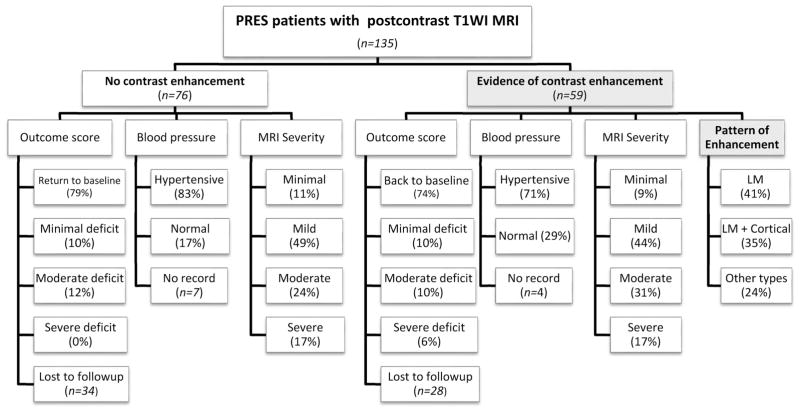
Of the 176 patient records reviewed, 41 patients were excluded from the dedicated MR imaging review due to the lack of post-contrast T1WI. A summary of the 135 included patients is presented in the form of an organizational chart in Fig 1, illustrating the patient cohort, and particularly showing the percentage of patients with the various MR imaging severity grades (exemplified in Figs 2–5) and clinical outcome grades in both the contrast-enhancing and nonenhancing subgroups.
FIG 1.
Organizational chart of the makeup of the cohort for this study, including MR imaging severity and various clinical factors. LM indicates leptomeningeal.
FIG 2.
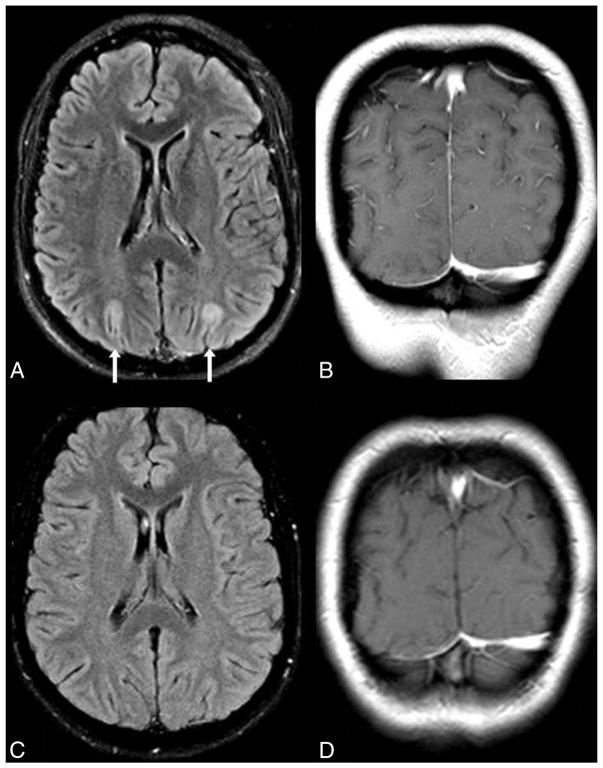
No enhancement in “minimal” PRES. A 22-year-old woman with end-stage renal disease presented with seizure, with SBPmax and DBPmax of 201/119 mm Hg within 1 day of MR imaging. “Minimal” cortical edema is noted on FLAIR (arrows, A) in the occipital regions, without abnormal contrast enhancement on postcontrast coronal T1WI (B). On a 3-month follow-up 1.5T MR imaging, the edema has resolved on FLAIR (C), and there is no enhancement on T1WI (D).
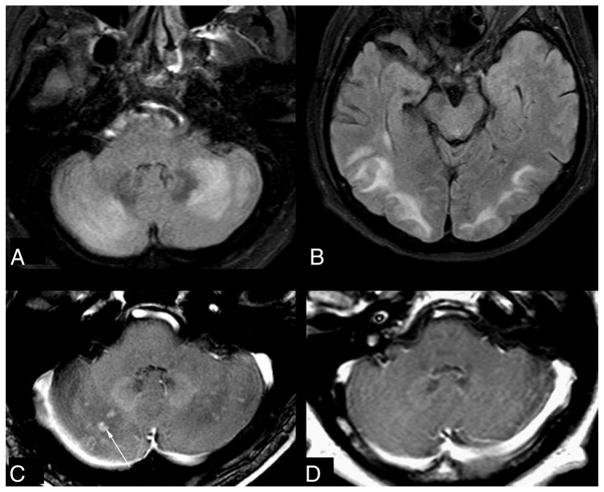
FIG 5.
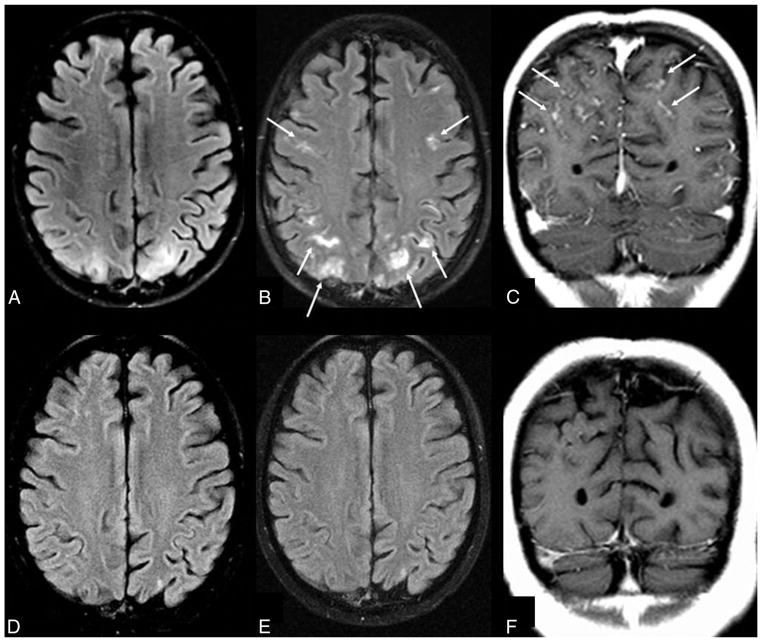
Leptomeningeal and cortical enhancement pattern in “severe” PRES. A 14-year-old girl with systemic lupus erythematosus presented with seizures. SBPmax and DBPmax were normal (136/83 mm Hg). The initial 1.5T MR imaging demonstrates severe edema on FLAIR (A), involving the basal ganglia, thalami, and brain stem (not shown), while there is diffusely thickened leptomeningeal enhancement (thin arrows) and cortical and parenchymal enhancement (dashed arrows) on postcontrast T1WI (B). Vessels are seen both within and outside of areas of vasogenic edema and remain an important consideration for these appearances, perhaps due to slow flow or endothelial dysfunction. On a follow-up 1.5T MR imaging 5 months later, the findings have entirely resolved on FLAIR (C) and postcontrast T1WI (D).
Clinical Findings
Of the 135 patients with PRES, the ages ranged from 7 to 82 years (mean, 40 ± 20 years; range, 3–80 years), 67.4% being female, as shown in Table 1. The most common primary attributable etiology of PRES was immunosuppressant or chemotherapeutic medications (n = 60), followed by the following: essential hypertensive emergency (n = 21), sepsis (n = 14), eclampsia (n = 12), systemic lupus erythematosus (n = 7), and multidrug use including cocaine abuse or alcohol withdrawal resulting in hypertensive crisis (n = 7). Other individual causes included dysautonomia, nortriptyline overdose, and adult respiratory distress syndrome, also resulting in hypertensive crisis (n = 4). In 10 patients, the cause of PRES was unclear.
Table 1.
Summary statistics for variables of interest by presence/absence of enhancement
| Variable and Category | Contrast Enhancement
|
P Value | |
|---|---|---|---|
| Positive (n = 59) | Negative (n = 76) | ||
| Sex | |||
| Male | 15 (25%) | 29 (38%) | .14 |
| Female | 44 (75%) | 47 (62%) | |
| Age | |||
| Median | 43 | 44 | .753 |
| Mean | 41 | 42 | |
| MRI severity | |||
| Minimal | 5 (8.5%) | 8 (10.5%) | .854 |
| Mild | 26 (44%) | 37 (48.7%) | |
| Moderate | 18 (30.5%) | 18 (23.7%) | |
| Severe | 10 (17%) | 13 (17.1%) | |
| Symptom | |||
| Seizure | 37 (62.7%) | 48 (63.2%) | .481 |
| AMS | 12 (20.3%) | 20 (26.3%) | |
| Others | 10 (17%) | 8 (10.6%) | |
| IPH | |||
| Yes | 8 (13.6%) | 6 (7.9%) | .395 |
| No | 51 (86.4%) | 70 (92.1%) | |
| SBPmax (mean) | 159.9 | 168.8 | .178 |
| DBPmax (mean) | 94.31 | 97.23 | .386 |
| Outcome score | |||
| Missing +4 | 28 | 34 | |
| 0 | 23 (74.2%) | 33 (78.6%) | .522 |
| 1 | 3 (9.7%) | 4 (9.5%) | |
| 2 | 3 (9.7%) | 5 (11.9%) | |
| 3 | 2 (6.5%) | 0 | |
Note:—IPH indicates intraparenchymal hemorrhage; AMS, altered mental status.
The recorded SBPmax and DBPmax within 1 day before and after the reference MR imaging were available in 124 patients; of these, 23% were normotensive (n = 29). The most common primary presenting symptom was seizures, present in 63% (n =85), followed by altered mental status in 24% (n =32). Other primary presenting symptoms included focal neurologic deficits (8.8%, n = 12) and headaches (4.4%, n = 6).
Clinical follow-up was available in 96 patients. In the remaining 39, no follow-up was available from the medical records. In 58% of the patients with a clinical follow-up (n = 56), there was a return to clinical baseline with no persistent neurologic deficit at follow-up, 7% had minimal residual neurologic deficit (n = 7), 8% had mild persistent neurologic deficits (n = 8), and 2% had a moderate persistent neurologic deficit (n = 2). Five percent of patients re-presented with seizures (n = 5). Nineteen percent of patients died in the short-term (n = 18), though only 1 patient died with causes attributable to PRES/seizures.
Radiologic Findings
Of the 135 included patients, 59 (43.7%) demonstrated evidence of contrast enhancement, 75% of these patients being females as shown in Table 2. Figure 2 shows an example of a case of PRES without evidence of enhancement, a pattern found in 76 patients (56.3%). The most common pattern of enhancement was leptomeningeal, identified in 76% (n = 45, Fig 3), whether isolated (41%, n = 24) or combined with a purely cortical pattern of enhancement (35%, n = 21; Fig 5). A purely nodular pattern of enhancement was visible in 3 patients (Fig 4), while 3 others exhibited both nodular and leptomeningeal patterns. Within the group of patients positive for contrast enhancement, 14% had radiologic evidence of intraparenchymal hemorrhage (n = 8) versus 8% in the group without evidence of intraparenchymal hemorrhage (n = 6). When we compared radiologic severity, patients with evidence of contrast enhancement were graded with minimal severity in 8% of cases (n = 5), mild in 44% (n = 26), moderate in 31% (n = 18), and severe in 17% (n = 10) versus 11%, 49%, 24%, and 17% (n = 8, 37, 18, 13), respectively, in the group of patients with no evidence of enhancement.
Table 2.
Summary statistics for variables of interest by pattern of contrast enhancement
| Variable and Category | Negative (n = 76) | Contrast Enhancement | Others (n = 14) | P Value | |
|---|---|---|---|---|---|
|
| |||||
| LM (n = 24) | Cortical + LM (n = 21) | ||||
| Sex | |||||
| Male | 29 (38.2%) | 4 (16.7%) | 5 (23.8%) | 6 (42.9%) | .154 |
| Female | 47 (61.8%) | 20 (83.3%) | 16 (76.2%) | 8 (57.1%) | |
| Age | |||||
| Median | 44 | 45 | 24 | 57 | .011 |
| Mean | 42 | 40 | 32 | 55 | |
| MRI severity | |||||
| Minimal | 8 (10.5%) | 2 (8.3%) | 2 (9.5%) | 1 (7.1%) | .942 |
| Mild | 37 (48.7%) | 10 (41.7%) | 10 (47.6%) | 6 (42.9%) | |
| Moderate | 18 (23.7%) | 6 (25%) | 6 (28.6%) | 6 (42.9%) | |
| Severe | 13 (17.1%) | 6 (25%) | 3 (14.3%) | 1 (7.1%) | |
| Symptom | |||||
| Seizure | 48 (63.2%) | 12 (50%) | 16 (76.2%) | 9 (64.3%) | .322 |
| AMS | 20 (26.3%) | 5 (20.8%) | 3 (14.3%) | 4 (28.6%) | |
| Others | 8 (10.5%) | 7 (29.2%) | 2 (9.5%) | 1 (7.1%) | |
| IPH | |||||
| Yes | 6 (7.9%) | 5 (20.8%) | 1 (4.8%) | 2 (14.3%) | .233 |
| No | 70 (92.1%) | 19 (79.2%) | 20 (95.2%) | 12 (85.7%) | |
| SBPmax (mean) | 168.8 | 166.7 | 142.8 | 172.8 | .037 |
| DBPmax (mean) | 97.23 | 96.74 | 88.11 | 99.08 | .260 |
| Outcome score | |||||
| Missing +4 | 34 | 13 | 7 | 8 | |
| 0 | 33 (78.6%) | 8 (72.7%) | 10 (71.4%) | 5 (83.3%) | .305 |
| 1 | 4 (9.5%) | 1 (9.1%) | 2 (14.3%) | 8 | |
| 2 | 5 (11.9%) | 0 | 2 (14.3%) | 1 (16.7%) | |
| 3 | 0 | 2 (18.2%) | 0 | 0 | |
Note:—LM indicates leptomeningeal; IPH, intraparenchymal hemorrhage; AMS, altered mental status.
FIG 3.
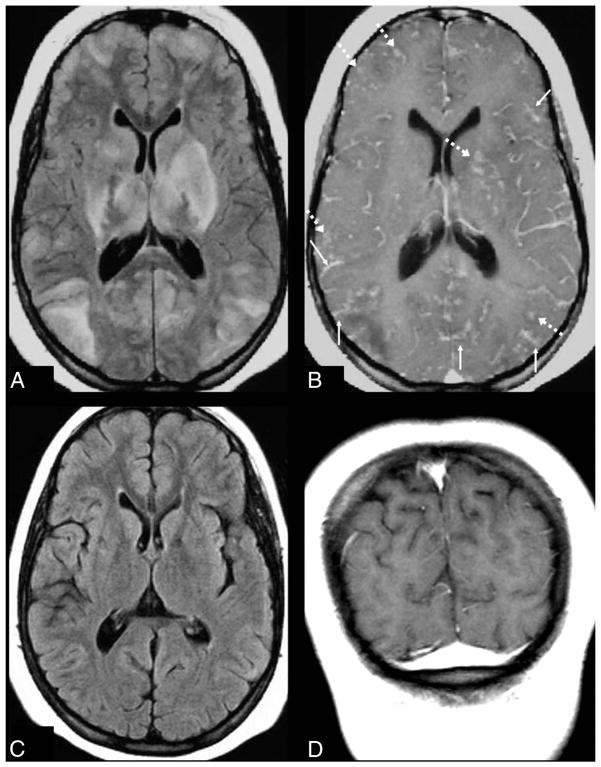
Leptomeningeal enhancement pattern in “mild” PRES. A 19-year-old woman with a history of systemic lupus erythematosus and pancytopenia presented with a seizure (blood pressure unavailable). A 1.5T MR imaging demonstrates mild parieto-occipital edema on FLAIR (A), with moderate leptomeningeal enhancement (thin arrows) on both gadolinium-enhanced FLAIR (B) and T1WI (C). D–F, A follow-up MR imaging 2 months later shows that both the mild cortical and subcortical edema on FLAIR (D) has resolved as well as the leptomeningeal enhancement on gadolinium-enhanced FLAIR (E) and T1WI (F). While gadolinium-enhanced FLAIR was not used to score the degree of edema or enhancement, the use of postcontrast FLAIR in this example demonstrates how enhancement can occur in areas lacking edema on noncontrast FLAIR, perhaps due to transient blood-brain barrier injury.
FIG 4.
Nodular enhancement pattern in “moderate” PRES. A 58-year-old man, on a multiple chemotherapy regimen for metastatic renal cancer with sepsis, developed seizures. The patient was hypotensive, with SBPmax and DBPmax of 92/67 mm Hg. The initial 3T MR imaging demonstrates moderate edema from PRES, graded moderate due to the degree of cerebellar and parieto-occipital edema on FLAIR (A and B); there is also nodular enhancement on postcontrast T1WI (C), demonstrated in multiple planes. D, On a follow-up MR imaging 7 days later, the enhancing cerebellar nodular lesions have resolved.
No significant difference was found between the presence of contrast enhancement and any of the tested variables (Table 1). When enhancement was present, an association was suggested between increased age and SBPmax and one of the described patterns of enhancement (P =.011 and .037, respectively) (Table 2). However, pair-wise comparison after Bonferroni correction (for P =.05/3) rejected this hypothesis for SBPmax(P = .029), whereas with respect to age, it failed to demonstrate which group was responsible for this result (leptomeningeal pattern versus cortical + leptomeningeal pattern groups, P =.187; cortical + leptomeningeal pattern versus other types of enhancement, P = .002; leptomeningeal versus other types of enhancement, P = .038). This analysis was thus interpreted as a type I error.
A statistically significant association between radiologic severity and clinical outcomes was found (P = .026), as shown in Table 3.
Table 3.
Association between radiologic severity and clinical outcome scorea
| Radiologic Severity | Outcome Score | |||
|---|---|---|---|---|
|
| ||||
| 0 | 1 | 2 | 3 | |
| Minimal | 8 (11%) | 0 | 0 | 0 |
| Mild | 29 (39.7%) | 3 (4.1%) | 2 (2.7%) | 0 |
| Moderate | 14 (19.2%) | 3 (4.1%) | 2 (2.7%) | 0 |
| Severe | 5 (6.9%) | 1 (1.4%) | 4 (5.5%) | 2 (2.7%) |
P value from the Fisher exact test = .026.
DISCUSSION
This study set out to determine the significance of contrast enhancement in PRES and to determine whether there was any association between the presence or type of enhancement and various clinical factors, including etiology, sex, maximum systolic or diastolic blood pressure, or clinical outcome. No such associations were evident between those recorded clinical factors and enhancement, suggesting that the presence or absence of enhancement does not affect prognosis. Also no association was found between the MR imaging severity score or the presence of hemorrhage and the presence of enhancement, which corroborates the findings of a prior study.7 Hence, intravenous gadolinium-based contrast is likely not necessary to evaluate the severity or extent of PRES, though studies with control groups would be necessary to truly prove this finding. The utility of postcontrast imaging would be in situations in which etiologies other than PRES are important considerations in the differential diagnosis, such as infectious meningitis in an immunocompromised patient, subacute phase of posterior circulation infarctions (especially if bilateral), or vasculitis/cerebritis; such entities could also exhibit leptomeningeal or cortical enhancement.
How does enhancement fit into the pathophysiology of PRES, if at all? Enhancement is generally considered to represent breakdown or increased permeability of the blood-brain barrier.17 The lack of a statistically significant pattern of enhancement with the different etiologies is of uncertain significance, and the pathophysiologic mechanism for these patterns of enhancement remains unclear. The presence or absence of enhancement could indicate different stages in the integrity of the blood-brain barrier, perhaps even being a temporal phenomenon, with cases lacking enhancement possibly being in a later stage at a point when the barrier has regained impermeability. By this rationale, a high incidence of enhancement would be expected in patients who are receiving drugs that are directly toxic to the endothelium, such as immunosuppressants.7,18,19 In this regard, 50% of the patients who were immunosuppressed in this study (who had received cyclosporine, tacrolimus, interferon, or mycophenolate) demonstrated at least 1 of the patterns of contrast enhancement, a finding not described previously, to our knowledge. The presence of increased permeability of the blood-brain barrier in certain etiologies or MR imaging patterns of PRES could perhaps be further evaluated by dynamic susceptibility contrast MR perfusion, perhaps with measurement of a permeability leakage coefficient such as K2, but this evaluation is beyond the scope of the current study. In this regard, though, a prior study by Brubaker et al20 did describe a normal K2 compared with controls, but that study comprised only 8 patients with PRES; thus, it may not have been large enough to adequately evaluate this phenomenon.21 Therefore, overall, the significance of contrast enhancement in PRES remains unclear but appears related to a transient impairment in function of the blood-brain barrier.
The traditional theory regarding the underlying pathophysiology of PRES is that a failure of cerebral autoregulation leads to a state of cerebral hyperperfusion through which the blood-brain barrier becomes permeable and that the resultant extravasation of macromolecules and other changes in the extracellular environment of the brain may induce seizures.22–26 A second theory is that hypoperfusion from exaggerated vasoconstriction/vasospasm as part of an autoregulatory mechanism leads to ischemia, followed by edema, with the ischemic changes affecting endothelial function and thus blood-brain barrier integrity. Accordingly, most studies of patients with PRES by using MR perfusion or hexamethylpropyleneamine oxime SPECT noted focal regions of reduced perfusion, with decreased cerebral blood flow.10,20,27–29 Another study did demonstrate rather focal areas of increased flow on hexamethylpropyleneamine oxime SPECT, though in a single patient of 2 included in the study, perhaps due to comorbidities or the delayed timing of the scan and concomitant therapeutic institution.30 A more recently proposed theory has been that of endothelial dysfunction due to a multitude of potential causes. Accordingly, recent studies of the effects of immunosuppressant medications on the endothelium suggest that endothelial cell injury and subsequent blood-brain barrier impairment may cause edema and microhemorrhage.7,18,19,31,32 Such impairment may explain the high rate of contrast enhancement, which often reverses, and may also explain the high incidence of microhemorrhages, which are seen in up to half of patients on SWI.32 The fact that both enhancement and microhemorrhages occur in normotensive patients from a variety of etiologies suggests that the mechanisms of hyperperfusion or hypoperfusion are not comprehensive explanations and that endothelial injury is more likely a common thread.1,7,10,13,19,31,32
The current study found the frequency of enhancement to be approximately 44%, previously reported to be 21%–38%.7,8 Such discrepancies among studies could relate to varying statistical power or varying reviewer sensitivities to contrast enhancement. As to differences in the composition of etiologies, the largest subset was patients with immunosuppression, showing similar percentages (44% in the current study; 45% and 50% in a study by Fugate et al8 and McKinney et al,7 respectively). Thus, it is unlikely that the makeup of etiologies accounts for differences in the described rates of enhancement.
In attributing a severity grade to the MR images of the patients in our cohort, we attempted, as a secondary end point, to associate the radiologic severity with clinical outcomes. A significant statistical association was found (P = .0255). Covarrubias et al3 previously demonstrated an association between poor clinical outcome and the extent of T2 signal abnormalities. This statistical association further corroborates this finding and lends credence to the idea of using an MR imaging severity grading system, as used in the current and prior studies, to quantify radiologic severity. Such descriptions of MR imaging severity could eventually aid in determining the prognosis of patients with PRES.3,7
Even though this study includes one of the largest cohorts of patients with PRES in the literature, it remains limited by being a retrospective study, with data flaws including the absence of or less well-defined clinical outcomes for some patients and the lack of consistent follow-up in all patients. In several patients, clinical outcome may have been biased by the existence of concomitant pathologies at the time of the diagnosis of PRES, including the presence of intracranial hemorrhage and associated brain infarction.
CONCLUSIONS
PRES can be readily identified on standard MR imaging, and contrast enhancement is not necessary during the evaluation of suspected PRES because it is associated with neither the MR imaging severity nor the clinical outcome. Additionally, other clinical factors such as presenting symptoms, age, sex, maximum blood pressure (systolic or diastolic), and etiology are not associated with the presence or pattern of contrast enhancement. A prospective study would better confirm these findings. The use of intravenous contrast may, nevertheless, be helpful in the evaluation of differential diagnoses. However, this study does suggest a strong association between the radiologic severity and clinical outcome and brings value to the use of an MR imaging grading scale in the prognosis of patients with PRES to estimate its reversibility.
ABBREVIATIONS
- DBPmax
maximum diastolic blood pressure
- PRES
posterior reversible encephalopathy syndrome
- SBPmax
maximum systolic blood pressure
Footnotes
Paper previously presented at: American Society of Neuroradiology Annual Meeting and the Foundation of the ASNR Symposium, April 25–30, 2015; Chicago, Illinois.
Disclosures: Alexander M. McKinney—UNRELATED: Consultancy: Vital Images, Minnetonka, Minnesota (a division of Toshiba Medical); CVRx (Brooklyn Park, Minnesota), Comments: <$5000 total reimbursement per year from Vital Images, <$500 total reimbursement per year from CVRx.
References
- 1.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29:1036–42. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 3.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23:1038–48. [PMC free article] [PubMed] [Google Scholar]
- 4.McKinney AM, Kieffer SA, Paylor RT, et al. Acute toxic leukoencephalopathy: potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. AJR Am J Roentgenol. 2009;193:192–206. doi: 10.2214/AJR.08.1176. [DOI] [PubMed] [Google Scholar]
- 5.Larsson HB, Stubgaard M, Frederiksen JL, et al. Quantitation of blood-brain barrier defect by magnetic resonance imaging and gadolinium-DTPA in patients with multiple sclerosis and brain tumors. Magn Reson Med. 1990;16:117–31. doi: 10.1002/mrm.1910160111. [DOI] [PubMed] [Google Scholar]
- 6.Stone LA, Smith ME, Albert PS, et al. Blood-brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing-remitting multiple sclerosis: relationship to course, gender, and age. Neurology. 1995;45:1122–26. doi: 10.1212/wnl.45.6.1122. [DOI] [PubMed] [Google Scholar]
- 7.McKinney AM, Short J, Truwit CL, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189:904–12. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 8.Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–32. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey SO, Sampaio RC, Michel E, et al. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 2000;21:1199–206. [PMC free article] [PubMed] [Google Scholar]
- 10.Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29:447–55. doi: 10.3174/ajnr.A0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones BV, Egelhoff JC, Patterson RJ. Hypertensive encephalopathy in children. AJNR Am J Neuroradiol. 1997;18:101–06. [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz RB, Feske SK, Polak JF, et al. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371–76. doi: 10.1148/radiology.217.2.r00nv44371. [DOI] [PubMed] [Google Scholar]
- 13.Rykken JB, McKinney AM. Posterior reversible encephalopathy syndrome. Semin Ultrasound CT MR. 2014;35:118–35. doi: 10.1053/j.sult.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Ugurel MS, Hayakawa M. Implications of post-gadolinium MRI results in 13 cases with posterior reversible encephalopathy syndrome. Eur J Radiol. 2005;53:441–49. doi: 10.1016/j.ejrad.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee P, McKinstry RC. Reversible posterior leukoencephalopathy syndrome: evaluation with diffusion-tensor MR imaging. Radiology. 2001;219:756–65. doi: 10.1148/radiology.219.3.r01jn48756. [DOI] [PubMed] [Google Scholar]
- 16.McKinney AM, Lohman BD, Sarikaya B, et al. Acute hepatic encephalopathy: diffusion-weighted and fluid-attenuated inversion recovery findings, and correlation with plasma ammonia level and clinical outcome. AJNR Am J Neuroradiol. 2010;31:1471–79. doi: 10.3174/ajnr.A2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27:525–51. doi: 10.1148/rg.272065155. [DOI] [PubMed] [Google Scholar]
- 18.Wilasrusmee C, Da Silva M, Singh B, et al. Morphological and biochemical effects of immunosuppressive drugs in a capillary tube assay for endothelial dysfunction. Clin Transplant. 2003;17:6–12. doi: 10.1034/j.1399-0012.17.s9.1.x. [DOI] [PubMed] [Google Scholar]
- 19.Benigni A, Morigi M, Perico N, et al. The acute effect of FK506 and cyclosporine on endothelial cell function and renal vascular resistance. Transplantation. 1992;54:775–80. doi: 10.1097/00007890-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Brubaker LM, Smith JK, Lee YZ, et al. Hemodynamic and permeability changes in posterior reversible encephalopathy syndrome measured by dynamic susceptibility perfusion-weighted MR imaging. AJNR Am J Neuroradiol. 2005;26:825–30. [PMC free article] [PubMed] [Google Scholar]
- 21.Weisskoff RM, Boxerman JL, Sorensen AG, et al. Simultaneous blood volume and permeability mapping using a single Gd-based contrast injection. Proceedings of the Second Meeting of the International Society of Magnetic Resonance in Medicine; San Francisco, California. August 6–12, 1994; p. 279. [Google Scholar]
- 22.MacKenzie ET, Strandgaard S, Graham DI, et al. Effects of acutely induced hypertension in cats on pial arteriolar caliber, local cerebral blood flow, and the blood-brain barrier. Circ Res. 1976;39:33–41. doi: 10.1161/01.res.39.1.33. [DOI] [PubMed] [Google Scholar]
- 23.Ijima T, Kubota Y, Kuroiwa T, et al. Blood-brain barrier opening following transient reflex sympathetic hypertension. Acta Neurochir Suppl. 1994;60:142–44. doi: 10.1007/978-3-7091-9334-1_38. [DOI] [PubMed] [Google Scholar]
- 24.Ivens S, Gabriel S, Greenberg G, et al. Blood-brain barrier breakdown as a novel mechanism underlying cerebral hyperperfusion syndrome. J Neurol. 2010;257:615–20. doi: 10.1007/s00415-009-5384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchi N, Angelov L, Masaryk T, et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–42. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–36. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez-Carpintero R, Narbona J, López de Mesa R, et al. Transient posterior encephalopathy induced by chemotherapy in children. Pediatr Neurol. 2001;24:145–48. doi: 10.1016/s0887-8994(00)00242-3. [DOI] [PubMed] [Google Scholar]
- 28.Naidu K, Moodley J, Corr P, et al. Single photon emission and cerebral computerised tomographic scan and transcranial Doppler sonographic findings in eclampsia. Br J Obstet Gynaecol. 1997;104:1165–72. doi: 10.1111/j.1471-0528.1997.tb10941.x. [DOI] [PubMed] [Google Scholar]
- 29.Engelter ST, Petrella JR, Alberts MJ, et al. Assessment of cerebral microcirculation in a patient with hypertensive encephalopathy using MR perfusion imaging. AJR Am J Roentgenol. 1999;173:1491–93. doi: 10.2214/ajr.173.6.10584788. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz RB, Jones KM, Kalina P, et al. Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992;159:379–83. doi: 10.2214/ajr.159.2.1632361. [DOI] [PubMed] [Google Scholar]
- 31.Zoja C, Furci L, Ghilardi F, et al. Cyclosporin-induced endothelial cell injury. Lab Invest. 1986;55:455–62. [PubMed] [Google Scholar]
- 32.McKinney AM, Sarikaya B, Gustafson C, et al. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33:896–903. doi: 10.3174/ajnr.A2886. [DOI] [PMC free article] [PubMed] [Google Scholar]