Abstract
Cartilage injuries caused by arthritis or trauma pose formidable challenges for effective clinical management due to the limited intrinsic proliferative capability of chondrocytes. Autologous stem cell-based therapies and transgene-enhanced cartilage tissue engineering may open new avenues for the treatment of cartilage injuries. Bone morphogenetic protein 2 (BMP2) induces effective chondrogenesis of mesenchymal stem cells (MSCs) and can thus be explored as a potential therapeutic agent for cartilage defect repair. However, BMP2 also induces robust endochondral ossification. Although the precise mechanisms through which BMP2 governs the divergence of chondrogenesis and osteogenesis remain to be fully understood, blocking endochondral ossification during BMP2-induced cartilage formation may have practical significance for cartilage tissue engineering. Here, we investigate the role of Sox9-donwregulated Smad7 in BMP2-induced chondrogenic differentiation of MSCs. We find that overexpression of Sox9 leads to a decrease in BMP2-induced Smad7 expression in MSCs. Sox9 inhibits BMP2-induced expression of osteopontin while enhancing the expression of chondrogenic marker Col2a1 in MSCs. Forced expression of Sox9 in MSCs promotes BMP2-induced chondrogenesis and suppresses BMP2-induced endochondral ossification. Constitutive Smad7 expression inhibits BMP2-induced chondrogenesis in stem cell implantation assay. Mouse limb explant assay reveals that Sox9 expands BMP2-stimulated chondrocyte proliferating zone while Smad7 promotes BMP2-intitated hypertrophic zone of the growth plate. Cell cycle analysis indicates that Smad7 induces significant early apoptosis in BMP2-stimulated MSCs. Taken together, our results strongly suggest that Sox9 may facilitate BMP2-induced chondrogenesis by downregulating Smad7, which can be exploited for effective cartilage tissue engineering.
Keywords: Sox9, Smad7, bone morphogenetic protein 2 (BMP2), mesenchymal stem cells (MSCs), chondrogenic differentiation, endochondral ossification, cartilage tissue engineering
INTRODUCTION
There have been currently limited techniques available to effectively repair cartilage injuries caused by osteoarthritis or trauma although cartilage restoration techniques have been explored for cartilage injury repair.1–4 The efficacy of cartilage restoration techniques, such as autologous chondrocyte implantation and osteochondral allograft, is largely limited by insufficient cell supplies, adverse donor site effects, and immunological reactions.5–7 Autologous stem cell and/or gene-based cartilage tissue engineering techniques may open new avenues for the treatment of cartilage injuries.1,4,7,8
Bone morphogenetic protein 2 (BMP2), a member of the TGFβ/BMP superfamily, regulates multiple aspects of chondrogenesis 9–11. BMP2 can induce chondrogenic differentiation of mesenchymal stem cells (MSCs), such as human bone marrow-derived stromal cells (hBMSCs), adipose-derived stem cells (ADSCs), mouse embryonic fibroblasts (MEFs) 4,12–17. During development, BMP2 promotes condensation, chondrogenic differentiation, chondrocyte proliferation, and hypertrophic differentiation4,16,18,19. Thus, BMP2 is capable of inducing both chondrogenic differentiation and endochondral ossification, which impairs cartilage formation by inducing apoptosis and degeneration 9,10,19–21. In fact, BMP2 was approved by the United States Food and Drug Administration (FDA) for promoting bone regeneration in spinal fusion and nonunion fracture healing 10,11,22. Thus, it is conceivable that BMP2 may be used for cartilage injury repair if BMP2-induced chondrogenesis in MSCs is enhanced and/or BMP2-induced osteogenesis is inhibited.
We have recently shown that exogenous expression of Sox9, an essential regulator of chondorgenesis4,8,16,23,24, is beneficial for cartilage formation through enhancing BMP2-induced chondrogenic differentiation and inhibiting ossification 25. However, Sox9 alone fails to induce chondrongenic differentiation of MSCs 25,26. These findings indicate that Sox9 may coordinate BMP2 signaling activity in regulating chondrogenic differentiation. However, it is not clear how Sox9 regulates BMP2-induced chondrogenic differentiation in MSCs.
Here we investigated the effect of Sox9-donwregulated Smad7 on BMP2-induced chondrogenic differentiation of MSCs. Smad7 belongs to the inhibitory Smad family and is an intracellular inhibitor of the TGFβ/BMP pathway10,27–29. We found that overexpression of Sox9 led to a decrease in BMP2-induced Smad7 expression in MSCs. Forced expression of Sox9 promoted BMP2-induced chondrogenesis and suppressed BMP2-induced endochondral ossification, while constitutive Smad7 expression inhibited BMP2-induced chondrogenesis in vivo. Mouse limb explant assay revealed that Sox9 expanded BMP2-stimulated proliferating zone while Smad7 promoted BMP2-intitated hypertrophic zone of the growth plate. Furthermore, Smad7 induced significant early apoptosis in BMP2-stimulated MSCs. These results strongly suggest that Sox9 may facilitate BMP2-induced chondrogenesis by downregulating Smad7, which can be exploited for effective cartilage tissue engineering.
MATERIALS AND METHODS
Cell culture and chemicals
HEK-293 and C3H10T1/2 cell lines were obtained from ATCC (Manassas, VA) and maintained in a complete Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in humidified 5% CO2 as reported30,31. Unless indicated otherwise, all other reagents were purchased from MilliporeSigma (St. Louis, MO) or Thermo-Fisher Scientific (Waltham, MA).
Recombinant adenoviruses expressing GFP, BMP2, Sox9, and Smad7
All the recombinant adenoviruses were constructed with AdEasy technology as described32,33. Briefly, the coding regions of human BMP2, mouse Smad7, and human Sox9 were PCR amplified and subcloned into adenoviral shuttle vectors, which were subsequently subjected to generating recombinant adenoviruses in HEK-293 or 293 derivatives 293pTP and RAPA lines 34,35, resulting Ad-BMP2, Ad-Smad7, and Ad-Sox9, which also co-express GFP. Ad-GFP was used as a mock virus control as described 36,37. For all adenoviral infections, polybrene (4–8μg/ml) was added to potentiate infection efficiency as previously described 38. Detailed information about vector constructions is available upon request.
Micromass plating and adenovirus infection of MSCs
Subconfluent MSCs were infected with Ad-GFP, Ad-BMP2, Ad-Sox9, Ad-Smad7, Ad-BMP2 and Ad-Sox9, respectively. At 24h after infection, the cells were collected and resuspended in culture medium at high-density (106 cells in a 50μl drop of media) as described 17,39. The cell droplet (50 μl) was added at the center of each well in the 12-well plate and then transferred to CO2 incubators for 2h, followed by adding 3ml of culture medium to each well. The medium was changed every 3 to 4 days. GFP expression was recorded at different time points.
RNA isolation and quantitative PCR (qPCR)
Total RNA was isolated by using TRIzol reagent (Thermo Fisher) and subjected to reverse transcription with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) as reported 36,40,41. The qPCR was carried out by using the SoAdvanced SYBR Green Supermix (Bio-Rad) under the following conditions: 95°C for 3min ×1 cycle; 95°C for 10 sec, 58°C for 5 sec ×40 cycles. The used qPCR primers include the following: Smad7 forward 5′-aag atc ggc tgt ggc atc-3″ and reverse 5′-cca aca gcg tcc tgg agt-3′; Col2a1 forward 5′-caa cac aat cca ttg cga ac-3′ and reverse 5′-tct gcc cag ttc agg tct ct-3′; Opn forward 5′-cct ccc ggt gaa agt gac-3′ and reverse 5′-ctg tgg cgc aag gag att-3′; and Gapdh forward 5′-cta cac tga gga cca ggt tgt ct-3′ and reverse 5′-ttg tca tac cag gaa atg agc tt-3′. The amplification efficiency and specificity of each primer set were confirmed by comparing the standard curves of the threshold cycle (Ct) values to the cDNA serial dilutions and melting curves. Gapdh was used a reference gene.
Western blot analysis
Cells were collected with the Lysis Buffer (Beyotime, China). The total cell lysate was cleared and subjected to 10% SDS-PAGE, followed by transfer to PVDF membranes. The membranes were blotted with the antibodies against Smad7, BMP2, GFP, or Sox9 (Santa Cruz Biotechnology Inc., Santa Cruz, USA), and subsequently probed with secondary antibodies conjugated with horseradish peroxidase (EarthOx, Millbrae, CA, USA). The proteins of interest were visualized by using the ECL detection kit (MilliporeSigma).
Ectopic cartilage and bone formation assay
The use and care of the animals and the animal experimental procedures were approved by the Ethical Committee of Chongqing Medical University, Chongqing, China. The ectopic bone formation assay was carried out as described42–44. Briefly, subconfluent C3H10T1/2 cells were infected with Ad-GFP, Ad-BMP2, Ad-Sox9, and/or Ad-Smad7. At 24h after infection, the cells were harvested and resuspended in PBS and injected subcutaneously (5×106 per injection) into the flanks of athymic nude (nu/nu) mice (5 mice per group, 6-wk old males; the Experimental Animal Center, Chongqing Medical University, Chongqing, China). The animals were maintained ad lib in the biosafety barrier facility. At 4 weeks after injection, the animals were euthanized. The ectopic masses were retrieved from injection sites and subjected to micro-CT imaging, followed by histological and other evaluations37,45.
Mouse fetal limb explant culture
Forelimbs of mouse embryos (E18.5) were dissected under sterile conditions and incubated in DMEM containing 0.5% FBS, 50 mg/ml ascorbic acid, 1 mM β-glycerophosphate, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C in humidified air with 5% CO2, for up to 14 days as described 46–48. At 12h after dissection the skin-free limbs were infected by adding Ad-GFP, Ad-BMP2, Ad-Sox9, and/or Ad-Smad7 to the culture medium. Medium was changed every 2 to 3 days. GFP signal was observed under a microscope to monitor the survival of the cells in the cultured tissues. At 14 days after culturing, the tissues were fixed for histological evaluation.
Hematoxylin and Eosin, Masson’s Trichrome and Alcian Blue staining
The retrieved and cultured tissues were fixed with 10% formalin, decalcified, and subjected to paraffin embedding. Serial sections at 5μm of embedded specimens were carried out, and mounted onto treated slides. The sections were deparaffinized and then rehydrated in a graduated fashion. H & E staining, Masson’s Trichrome staining, and Alcian Blue staining were done as described 49–51.
Apoptosis analysis
Subconfluent C3H10T1/2 cells were infected with Ad-BMP2, Ad-GFP, Ad-Smad7 and/or Ad-Sox9 and cultured for 3 days. The cells were harvested, washed with PBS, and resuspended in the binding buffer (100μl of calcium buffer containing 10mM HEPES, pH 7.4, 140mM NaCl, and 2.5mM CaCl2), followed by double staining with the APC Annexin V Apoptosis Detection Kit with 7-AAD (BioLegend, San Diego, CA, USA). The stained cells were subjected to flow cytometry to identify live cells (Annexin V-PE−/7-AAD−), early apoptotic cells (Annexin V-PE+/7-AAD−), late apoptotic or secondary apoptotic cells (Annexin V-PE+/7-AAD+), and necrotic cells (Annexin V-PE−/7-AAD+).
Statistical analysis
All quantitative experiments were performed in triplicate and/or repeated three independent experiments. Data were expressed as mean + standard deviation (SD). The one-way analysis of variance was used to analyze statistical significance. A value of p<0.05 was considered statistically significant.
RESULTS
Sox9 inhibits BMP2-induced Smad7 expression in MSCs
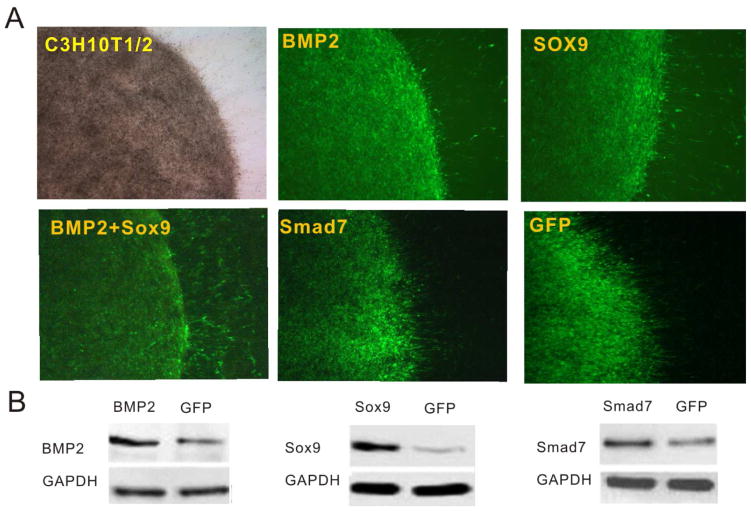
In order to achieve high levels of transgene expression we constructed a panel of recombinant adenoviruses expressing Smad7, BMP2, or Sox9. When C3H10T1/2 cells were seeded as micromass culture and infected with Ad-BMP2, Ad-Sox9, Ad-Smad7, Ad-BMP2+Ad-Sox9, or Ad-GFP, we found that micromasses were effectively transduced by the adenoviral vectors at 24h after infection (Figure 1A). Western blotting analysis conformed that the transgenes were highly expressed in the micromasses that were infected with respective adenoviral vectors for 48h (Figure 1B).
Figure 1. Adenovirus-mediated effective transduction of BMP2, Sox9, Smad7 and GFP into the micro-mass formed with C3H10T1/2 cells.
(A) C3H10T1/2 cells were seeded as micromasses and infected with Ad-BMP2, Ad-Sox9, Ad-BMP2+Ad-Sox9, Ad-Smad7 and Ad-GFP. Representative bright field and GFP fluorescence fields were recorded at 24h after infection. Representative results are shown. (B) Western blot analysis of adenovirus-mediated transgene expression. Total cell lysate was collected form the micromass culture at 48h after infection and subjected to SDS-PAGE. The transgene expression of BMP2, Sox9 and Smad7 was probed with respective antibodies. Ad-GFP infected cells were used as negative controls. GAPDH expression was used as loading controls. Representative results are shown.
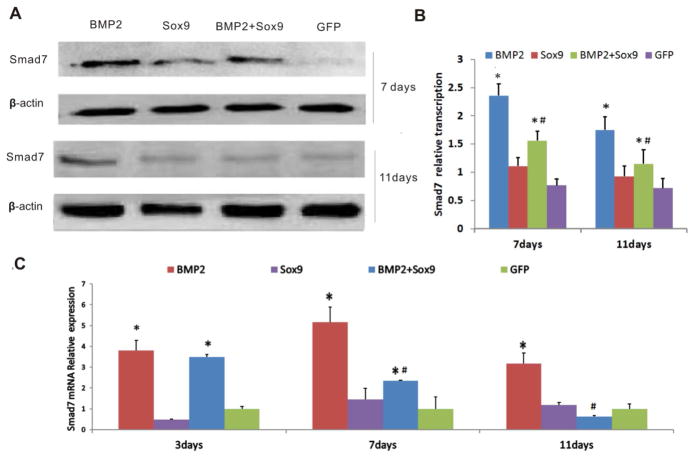
Using the adenoviral vectors, we tested whether exogenous expression of Sox9 would affect Smad7 level upon BMP2 stimulation in C3H10T1/2 cells. Western blotting analysis revealed that Smad7 was up-regulated by BMP2 while Sox9 alone did not significantly affect Smad7 expression, compared with that of the GFP alone group (Figure 2AB). However, constitutive Sox9 expression significantly inhibited BMP2-induced expression of Smad7, compared with that of the BMP2 alone groups (Figure 2AB). These results were further confirmed by qPCR analysis. We found that BMP2 effectively up-regulated Smad7 expression at days 3, 7 and 11 days after infection (Figure 2C), while BMP2-induced Smad7 expression was significantly inhibited by Sox9, especially at days 7 and 11 after infection (Figure 2C). These results strongly suggest that Sox9 may suppress Smad7 expression induced by BMP2 in MSCs.
Figure 2. Sox9 down-regulates BMP2-induced expression of Smad7 in MSCs.
(A & B) Western blotting analysis of Smad7 expression at protein level. C3H10T1/2 cells were infected with the indicated adenoviral vectors. At 7 and 11 days after infection, total cell lysate was prepared and subjected to Western blotting analysis with an anti-Smad7 antibody or anti-β-actin control (A). The Western blotting results were quantitatively analyzed (B). BMP2-induced Smad7 expression was higher than that in the GFP group (p<0.05). In the BMP2+Sox9 group, overexpression of Sox9 downregulated BMP2-induced Smad7 expression at days 7 and 11 compared to that of the BMP2 group (p<0.05). (C) qPCR analysis of Smad7 expression at mRNA level. C3H10T1/2 cells were infected with the indicated adenoviral vectors. Total RNA was isolated at 3, 7, and 11 days after infection and subjected to quantitative RT-PCR (qPCR) using Smad7-rescific primers. BMP2 upregulated Smad7 mRNA levels at days 3, 7, and 11 compared to the GFP group (p<0.05). At days 7 and 11 overexpression of Sox9 decreased BMP2-induced Smad7 mRNA level, compared to that of the BMP2 group (p<0.05). Gapdh was used as a reference gene. “*” p<0.05, vs. Ad-GFP; “#” p<0.05, vs. Ad-BMP2.
Co-expression of BMP2 and Sox9 promotes chondrogenic differentiation while inhibiting osteogenic markers in MSCs
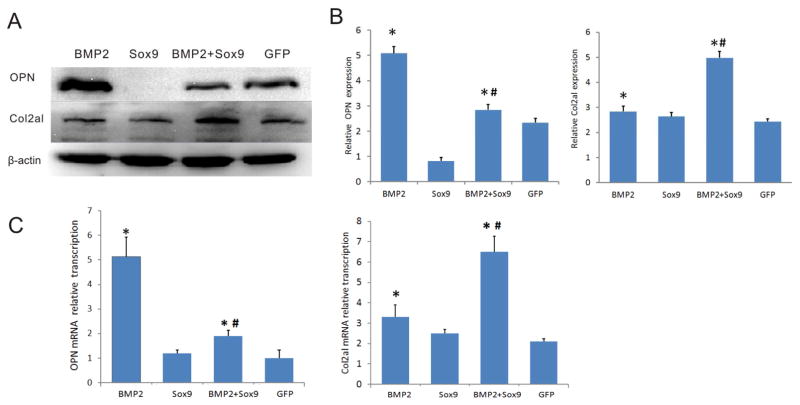
We further analyzed the effect on Sox9 on BMP2-induced expression of chondrogenic and osteogenic markers in MSCs. BMP2 was shown to effectively induce Opn expression, which Sox9 inhibited Opn expression (Figure 3AB). Furthermore, Sox9 was shown to significantly inhibit BMP2-induced Opn expression (Figure 3AB). Conversely, Sox9 was shown to enhance BMP2-induced expression of chondrogenic marker Col2a1 (Figure 3AB). Similar results were obtained from qPCR analysis. BMP2 was shown to induce Col2a1 expression, which was significantly enhanced by Sox9 (Figure 3C). On the contrary BMP2-induced Opn expression was drastically suppressed by Sox9 expression although BMP2 effectively up-regulated Opn expression (Figure 3C). Collectively, these results indicate that Sox9 can inhibit Smad7 expression and BMP2-induced endochondral ossification and hence promote BMP2-induced chondrogenic differentiation.
Figure 3. Sox9 inhibits BMP2-induced osteogenic marker, but potentiates BMP2-induced chondrogenic marker.
(A & B). Western blotting analysis of OPN and Col2aI expression. C3H10T1/2 cells were infected with the indicated adenoviral vectors. At 7 days after infection, total cell lysate was prepared and subjected to Western blotting analysis with an OPN, Col2al, or β-actin antibodies (A). Western blotting results were quantitatively analyzed by using Quantity one (B). (C). qPCR analysis of OPN and Col2aI expression. C3H10T1/2 cells were infected with the indicated adenoviral vectors. Total RNA was isolated at 5 days after infection and subjected to quantitative RT-PCR (qPCR) using Col2al and OPN-specific primers. Gapdh was used as a reference gene. “#” p<0.05, vs. Ad-BMP2; “*” p<0.05, vs. Ad-GFP group.
Sox9 impairs BMP2-induced ectopic ossification and promotes chondrogenesis, while Smad7 blocks BMP2-induced cartilage formation
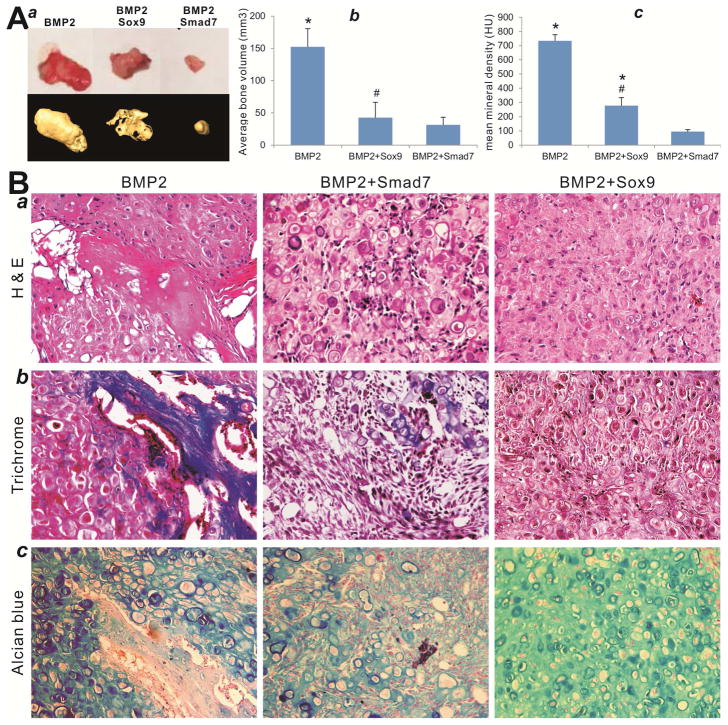
We next analyzed the in vivo effect of Sox9 and Smad7 on BMP2-induced ectopic bone and cartilage formation using our previously established stem cell implantation assay 45,49,52,53. When C3H10T1/2 cells were infected with Ad-GFP, Ad-BMP2, Ad-Sox9, and/or Ad-Smad7 and injected subcutaneously into the flanks of athymic nude mice for 4 weeks, we found that the cells transduced with Ad-GFP, AdSmad7 or AdSox9 alone failed to form any detectable masses (data not shown). The cells transduced by BMP2 alone formed the largest ectopic bone/cartilage masses, while BMP2+Smad7 group formed the smallest masses (Figure 4A-ab). The cells co-transduced with BMP2 and Sox9 formed significantly smaller masses than that of the BMP2 alone group (Figure 4A-ab). Furthermore, microCT imaging analysis indicates that the mean bone mineral density was significantly decreased in BMP2+Sox9 and BMP2+Smad7 groups, compared with that of the BMP2 only group (Figure 4A-c).
Figure 4. Sox9 promotes BMP2-induced chondrogenesis in MSCs, which is inhibited by Smad7.
(A) C3H10T1/2 cells were infected with the indicated adenoviral vectors and implanted subcutaneously in athymic nude mice for 8 weeks. The retrieved masses were subjected to microCT analysis (A-a) to determine the average bone volume (A-b) and the mean mineral density in Hounsfield unit (HU) (A-c). No masses were retrieved from Sox9 only, Smad7 only, and GFP only groups. Representative images are shown. “*”, p<0.05, vs. Ad-BMP2+Ad-GFP; “#” p<0.05, vs. Ad-BMP2+Ad-Sox9. (B). Histologic evaluation of the retrieved bone masses. The retrieved masses were fixed, decalcified and subjected to hematoxylin and eosin (H & E) (a), Masson’s trichrome (b), and alcian blue staining (c). Representative images are shown.
H & E staining indicates that BMP2 induced robust bone formation with traces of cartilage formation, which was significantly inhibited by Smad7 overexpression, while Sox9 overexpression effectively inhibited BMP2-induced bone formation and led to the formation of cartilage-like tissues (Figure 4C-a). Trichrome staining further confirmed that BMP2-induced ossification was completely inhibited by Sox9 overexpression (Figure 4C-b), while alcian blue staining analysis indicates that Sox9 significantly promoted BMP2-induced cartilage formation from MSCs (Figure 4C-c). These in vivo findings further confirm our in vitro results about the chondrogenesis-promoting effect of Sox9 on BMP2-induced differentiation of MSCs.
Smad7 promotes chondrocyte hypertrophy and apoptosis upon BMP2 stimulation, which is alleviated by Sox9
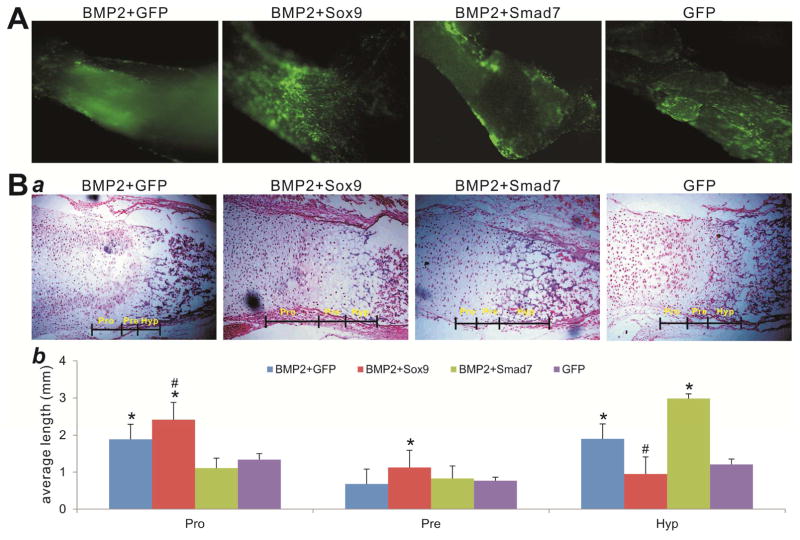
We further analyzed the interplay between Sox9 and Smad7 in the presence of BMP2 at growth plate using the fetal limb explant culture assay as described 46,54. The mouse E18.5 perinatal forelimbs were cultured and shown to be effectively co-transduced with AdGFP, AdBMP2 and/or Ad-GFP, Ad-Sox9, or Ad-Smad7 for up to 2 weeks (Figure 5A). At the endpoint we analyzed the cultured explants histologically and found that BMP2 promoted a coordinated expansion of cartilage zones at the growth plat, while Sox9 overexpression enhanced BMP2-induced expansion of proliferating chondrocyte zone (Figure 5BC). However, overexpression of Smad7 led to a significant expansion of the hypertrophic chondrocyte zone while decreasing the proliferating chondrocyte zone (Figure 5BC). These results suggest that Smad7 may impair BMP2-induced chondrocyte proliferation and promote chondrocyte hypertrophy in the fetal limb explant culture, which may be reversed by Sox9 overexpression.
Figure 5. Sox9 promotes BMP9-induced proliferation of chondrocytes while Smad7 promotes chondrocyte hypertrophy of mouse limb explants.
(A) Mouse limb explant culture. Mouse E18.5 forelimbs (n=4 per group) were cultured in an organ culture medium containing Ad-BMP2 and Ad-GFP, Ad-Smad7, or Ad-Sox9. The infection efficiency was visualized under a fluorescent microscope at 48h after infection. Representative images are shown. (B) Histological analysis of the cultured forelimbs. The forelimbs were subjected to H & E staining (a). Representative images are shown. Quantitative analysis of the average length of proliferating chondrocyte zone, hypertrophic zones, and pre-hypertrophic zones of the growth plate (b). The histologic stains were analyzed by using the Image J software. “Pro”, proliferative chondrocyte zone; “Hyp”, hypertrophic chondrocyte zone; “Pre”, pre-hypertrophic chondrocyte zone. “#” p<0.05, vs. Ad-BMP2+Smad7; “*” p<0.05, vs. Ad-GFP.
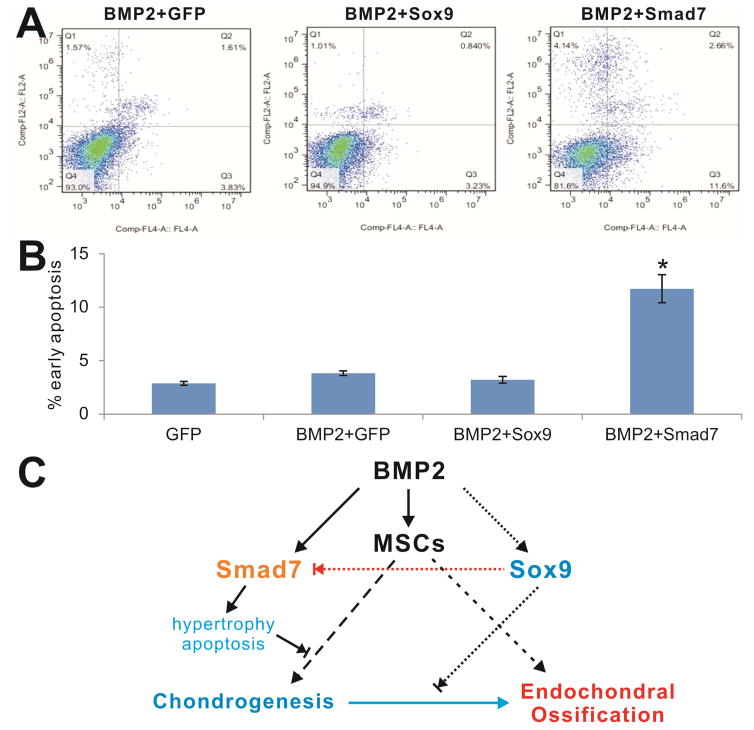
We performed flow cytometry analysis to evaluate the effect of Smad7 on the apoptosis in BMP2-stimulated MSCs. We found that Smad7 overexpression significantly increased the early apoptosis rate in BMP2-stimulated MSCs, compared with that of the control group or Sox9 group (Figure 6AB). These results suggest that Smad7 may induce chondrocyte apoptosis. Thus, downregulating Smad7 expression such as through Sox9 overexpression may represent a novel approach to promote BMP2-induced chondrogenesis from MSCs.
Figure 6. Sox9 alleviates Smad7-induced early apoptosis in BMP2-stimulated MSCs.
Subconfluent C3H10T1/2 cells were infected with the indicated adenoviral vectors for 3 days. The infected cells were collected and subjected to apoptosis assay (A). The early apoptosis rates were quantitatively assessed and graphed (B). “*”, p<0.001 vs. BMP2+GFP group. (C) A potential mechanism underlying how Sox9 may promote BMP2-induced chondrogenic differentiation through inhibiting Smad7.
DISCUSSION
BMP2 induces not only cartilage formation but also endochondral ossification although the precise mechanisms through which BMP2 governs the divergence of chondrogenesis and osteogenesis remain to be fully understood4,10,55. In this study, we found that overexpression of Sox9 can effectively suppress Smad7 expression in the later stage of chondrogenic differentiation even though Smad7 is upregulated by BMP2 during chondrogenesis. When Sox9 is overexpressed, Smad7 expression is downregulated and BMP2-induced chondrogenesis is concurrently enhanced in vitro and in vivo. Thus, our results strongly suggest that Sox9 may promote BMP2-induced chondrogenic differentiation through inhibiting Smad7 expression (Figure 6C).
As one of the two inhibitory Smad proteins, Smad7 seemingly plays a distinct role in BMP2-induced chondrogenic differentiation4. It was reported that BMP2-induced cartilaginous nodule formation was downregulated by Smad7, but not Smad6, through inhibiting the Smad1/5/8 and p38 pathway 27,56,57. Smad7 was shown more closely related to endochondral ossification as Smad7 knock-out mice exhibited ossification defects in the calvarial bones56. A partial loss of Smad7 function led to reduced osteogenic potential, fewer mineralized nodules, lower ALP activity, and reduced gene expression of osteogenic markers Col1A1, Runx2, and OCN58. Furthermore, it was reported that high levels of Smad7 induced by BMP2 may pose as a major obstacle to chondrogenic differentiation 27,28.
In this study, we have demonstrated that Sox9 may suppress Smad7 expression and hence promote BMP2-induced chondrogenesis (Figure 6C). Even though Sox9 is considered as a key transcription regulator for chondrogenesis4,59, many studies demonstrate that Sox9 alone is insufficient to chondrogenic differentiation from MSCs 4,60,61. Our results also revealed that exogenous expression of Sox9 failed to induce cartilage formation in stem cell implantation assay and had limited effect on inducing Col2al expression. Thus, it’s conceivable that Sox9 is more likely to cooperate with other regulators during BMP2-induced chondrogenic differentiation of MSCs.
While the exact mechanism underlying the role of Sox9 in BMP2-induced chondrogenesis remains unclear, downregulation of Smad7 may at least in part explain how Sox9 cooperates with BMP2 in cartilage formation. Overexpression of Sox9 during BMP2-induced chondrogenesis may up-regulate Sox5/Sox6/Sox9 and hence enhance chondrogenic differentiation62,63. We previously found that Runx2 can be downregulated by Sox9, which may lead to delayed ossification25. It was reported that a loss of Smad7 downregulated Runx2 expression58. Furthermore, BMP2 was shown to promote differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines19, suggesting that Runx2 may play a limited role during endochondral ossification. Nonetheless, a decrease in Smad7 may be beneficial for Sox9-promoting chondrogenic differentiation while blocking endochondral ossification in BMP2-transduced MSCs.
Blocking endochondral ossification during BMP2-induced cartilage formation may have practical significance for cartilage tissue engineering. During endochondral ossification chondrocytes in the centers of the cartilage condensations exit cell cycle and evolve into prehypertrophic, and then hypertrophic chondrocytes, which eventually undergo apoptosis. Subsequently, the cartilage matrix is invaded by blood vessels, osteoblasts, and osteoclasts, which degrade the cartilage matrix and replace cartilage with bone56. Smad7 expression is closely related with endochondral ossification and maturation of chondrocytes. Here, we demonstrated that Smad7 expression is suppressed by Sox9, resulting in diminished endochondral ossification and decreased apoptosis of chondrocytes.
In summary, we demonstrate that Sox9 can effectively down-regulate Smad7 expression, and hence cooperates with BMP2 during cartilage formation by enhancing chondrogenic differentiation and blocking endochondral ossification. Thus, it is conceivable that overexpression of Sox9 and/or inhibition of Smad7 may be exploited as novel strategies to develop more efficient cartilage tissue engineering to treat cartilage injuries.
Acknowledgments
We thank the technical assistance provided by Chongqing Key Laboratory of Clinical Hematology at Third Military Medical University. The reported work was supported in part by research grants from the Natural Sciences Foundation of China (#81572142 and #81371972 to WH), the National Institutes of Health (AT004418 to TCH), the US Department of Defense (OR130096 to JMW), the Scoliosis Research Society (TCH and MJL), and the 973 Program of the Ministry of Science and Technology (MOST) of China (# 2011CB707906 to TCH). The reported work was also supported in part by The University of Chicago Cancer Center Support Grant (P30CA014599) and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000430. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adachi N, Ochi M, Deie M, et al. Implantation of tissue-engineered cartilage-like tissue for the treatment for full-thickness cartilage defects of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1241–1248. doi: 10.1007/s00167-013-2521-0. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Merchan EC. The treatment of cartilage defects in the knee joint: microfracture, mosaicplasty, and autologous chondrocyte implantation. Am J Orthop (Belle Mead NJ) 2012;41(5):236–239. [PubMed] [Google Scholar]
- 3.Rodriguez-Merchan EC. Regeneration of articular cartilage of the knee. Rheumatol Int. 2013;33(4):837–845. doi: 10.1007/s00296-012-2601-3. [DOI] [PubMed] [Google Scholar]
- 4.Green JD, Tollemar V, Dougherty M, et al. Multifaceted signaling regulators of chondrogenesis: Implications in cartilage regeneration and tissue engineering. Genes Dis. 2015;2(4):307–327. doi: 10.1016/j.gendis.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchetina E, Mwale F, Poole AR. Distinct phases of coordinated early and late gene expression in growth plate chondrocytes in relationship to cell proliferation, matrix assembly, remodeling, and cell differentiation. J Bone Miner Res. 2003;18(5):844–851. doi: 10.1359/jbmr.2003.18.5.844. [DOI] [PubMed] [Google Scholar]
- 6.Benthien JP, Schwaninger M, Behrens P. We do not have evidence based methods for the treatment of cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):543–552. doi: 10.1007/s00167-010-1271-5. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery SR, Foster BD, Ngo SS, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070–2075. doi: 10.1007/s00167-013-2614-9. [DOI] [PubMed] [Google Scholar]
- 8.Jo A, Denduluri S, Zhang B, et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014;1(2):149–161. doi: 10.1016/j.gendis.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luu HH, Song WX, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 10.Wang RN, Green J, Wang Z, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Song J, Zhang H, et al. Wnt and BMP Signaling Crosstalk in Regulating Dental Stem Cells: Implications in Dental Tissue Engineering. Genes Dis. 2016;3(4):263–276. doi: 10.1016/j.gendis.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Kang Q, Sun MH, Cheng H, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 14.Rocha B, Calamia V, Mateos J, Fernandez-Puente P, Blanco FJ, Ruiz-Romero C. Metabolic labeling of human bone marrow mesenchymal stem cells for the quantitative analysis of their chondrogenic differentiation. J Proteome Res. 2012;11(11):5350–5361. doi: 10.1021/pr300572r. [DOI] [PubMed] [Google Scholar]
- 15.An C, Cheng Y, Yuan Q, Li J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng. 2010;38(4):1647–1654. doi: 10.1007/s10439-009-9892-x. [DOI] [PubMed] [Google Scholar]
- 16.Pan Q, Yu Y, Chen Q, et al. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J Cell Physiol. 2008;217(1):228–241. doi: 10.1002/jcp.21496. [DOI] [PubMed] [Google Scholar]
- 17.Lamplot JD, Liu B, Yin L, et al. Reversibly Immortalized Mouse Articular Chondrocytes Acquire Long-Term Proliferative Capability While Retaining Chondrogenic Phenotype. Cell Transplant. 2015;24(6):1053–1066. doi: 10.3727/096368914X681054. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Shi W, Cao X. Multiplicity of BMP signaling in skeletal development. Ann N Y Acad Sci. 2007;1116:29–49. doi: 10.1196/annals.1402.053. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Gao Y, Sakamoto K, et al. BMP-2 promotes differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines. J Cell Physiol. 2007;211(3):728–735. doi: 10.1002/jcp.20988. [DOI] [PubMed] [Google Scholar]
- 20.Shu B, Zhang M, Xie R, et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124(Pt 20):3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng ZL, Sharff KA, Tang N, et al. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 22.Mesfin A, Buchowski JM, Zebala LP, et al. High-dose rhBMP-2 for adults: major and minor complications: a study of 502 spine cases. J Bone Joint Surg Am. 2013;95(17):1546–1553. doi: 10.2106/JBJS.L.01730. [DOI] [PubMed] [Google Scholar]
- 23.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22(1):85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 24.Ikegami D, Akiyama H, Suzuki A, et al. Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development. 2011;138(8):1507–1519. doi: 10.1242/dev.057802. [DOI] [PubMed] [Google Scholar]
- 25.Liao J, Hu N, Zhou N, et al. Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PLoS One. 2014;9(2):e89025. doi: 10.1371/journal.pone.0089025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattori T, Muller C, Gebhard S, et al. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. 2010;137(6):901–911. doi: 10.1242/dev.045203. [DOI] [PubMed] [Google Scholar]
- 27.Iwai T, Murai J, Yoshikawa H, Tsumaki N. Smad7 Inhibits chondrocyte differentiation at multiple steps during endochondral bone formation and down-regulates p38 MAPK pathways. J Biol Chem. 2008;283(40):27154–27164. doi: 10.1074/jbc.M801175200. [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Bringas P, Jr, Mogharei A, Zhao J, Deng C, Chai Y. Receptor-regulated and inhibitory Smads are critical in regulating transforming growth factor beta-mediated Meckel’s cartilage development. Dev Dyn. 2002;224(1):69–78. doi: 10.1002/dvdy.10088. [DOI] [PubMed] [Google Scholar]
- 29.Sakou T, Onishi T, Yamamoto T, Nagamine T, Sampath T, Ten Dijke P. Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Res. 1999;14(7):1145–1152. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- 30.Liao J, Wei Q, Fan J, et al. Characterization of retroviral infectivity and superinfection resistance during retrovirus-mediated transduction of mammalian cells. Gene Ther. 2017;24(6):333–341. doi: 10.1038/gt.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao J, Wei Q, Zou Y, et al. Notch Signaling Augments BMP9-Induced Bone Formation by Promoting the Osteogenesis-Angiogenesis Coupling Process in Mesenchymal Stem Cells (MSCs) Cell Physiol Biochem. 2017;41(5):1905–1923. doi: 10.1159/000471945. [DOI] [PubMed] [Google Scholar]
- 32.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo J, Deng ZL, Luo X, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2(5):1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 34.Wu N, Zhang H, Deng F, et al. Overexpression of Ad5 precursor terminal protein accelerates recombinant adenovirus packaging and amplification in HEK-293 packaging cells. Gene Ther. 2014;21(7):629–637. doi: 10.1038/gt.2014.40. [DOI] [PubMed] [Google Scholar]
- 35.Wei Q, Fan J, Liao J, et al. Engineering the Rapid Adenovirus Production and Amplification (RAPA) Cell Line to Expedite the Generation of Recombinant Adenoviruses. Cell Physiol Biochem. 2017;41(6):2383–2398. doi: 10.1159/000475909. [DOI] [PubMed] [Google Scholar]
- 36.Fan J, Wei Q, Liao J, et al. Noncanonical Wnt signaling plays an important role in modulating canonical Wnt-regulated stemness, proliferation and terminal differentiation of hepatic progenitors. Oncotarget. 2017;8(16):27105–27119. doi: 10.18632/oncotarget.15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao J, Yu X, Hu X, et al. lncRNA H19 mediates BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) through Notch signaling. Oncotarget. 2017 doi: 10.18632/oncotarget.18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao C, Wu N, Deng F, et al. Adenovirus-mediated gene transfer in mesenchymal stem cells can be significantly enhanced by the cationic polymer polybrene. PLoS One. 2014;9(3):e92908. doi: 10.1371/journal.pone.0092908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shui W, Yin L, Luo J, et al. Characterization of chondrocyte scaffold carriers for cell-based gene therapy in articular cartilage repair. J Biomed Mater Res A. 2013;101(12):3542–3550. doi: 10.1002/jbm.a.34661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng Y, Zhang J, Wang Z, et al. Antibiotic monensin synergizes with EGFR inhibitors and oxaliplatin to suppress the proliferation of human ovarian cancer cells. Sci Rep. 2015;5:17523. doi: 10.1038/srep17523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang F, Li Y, Zhang H, et al. Anthelmintic mebendazole enhances cisplatin’s effect on suppressing cell proliferation and promotes differentiation of head and neck squamous cell carcinoma (HNSCC) Oncotarget. 2017;8(8):12968–12982. doi: 10.18632/oncotarget.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye J, Wang J, Zhu Y, et al. A thermoresponsive polydiolcitrate-gelatin scaffold and delivery system mediates effective bone formation from BMP9-transduced mesenchymal stem cells. Biomed Mater. 2016;11(2):025021. doi: 10.1088/1748-6041/11/2/025021. [DOI] [PubMed] [Google Scholar]
- 43.Lu M, Liao J, Dong J, et al. An effective treatment of experimental osteomyelitis using the antimicrobial titanium/silver-containing nHP66 (nano-hydroxyapatite/polyamide-66) nanoscaffold biomaterials. Sci Rep. 2016;6:39174. doi: 10.1038/srep39174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song D, Zhang F, Reid RR, et al. BMP9 induces osteogenesis and adipogenesis in the immortalized human cranial suture progenitors from the patent sutures of craniosynostosis patients. J Cell Mol Med. 2017 doi: 10.1111/jcmm.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Liao J, Zhang F, et al. NEL-Like Molecule-1 (Nell1) Is Regulated by Bone Morphogenetic Protein 9 (BMP9) and Potentiates BMP9-Induced Osteogenic Differentiation at the Expense of Adipogenesis in Mesenchymal Stem Cells. Cell Physiol Biochem. 2017;41(2):484–500. doi: 10.1159/000456885. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Jiang W, Huang J, et al. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25(11):2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Cui J, Zhang BQ, et al. Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J Biomed Mater Res A. 2014;102(4):1017–1025. doi: 10.1002/jbm.a.34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Z, Yin L, Wang Z, et al. A Novel Organ Culture Model of Mouse Intervertebral Disc Tissues. Cells Tissues Organs. 2016;201(1):38–50. doi: 10.1159/000439268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Wang J, Deng F, et al. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs) Biomaterials. 2015;39:145–154. doi: 10.1016/j.biomaterials.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Zhang H, Zhang W, et al. Bone morphogenetic protein-9 effectively induces osteo/odontoblastic differentiation of the reversibly immortalized stem cells of dental apical papilla. Stem Cells Dev. 2014;23(12):1405–1416. doi: 10.1089/scd.2013.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Hong S, Li M, et al. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res. 2013;31(11):1796–1803. doi: 10.1002/jor.22427. [DOI] [PubMed] [Google Scholar]
- 52.Wang N, Zhang W, Cui J, et al. The piggyBac transposon-mediated expression of SV40 T antigen efficiently immortalizes mouse embryonic fibroblasts (MEFs) PLoS One. 2014;9(5):e97316. doi: 10.1371/journal.pone.0097316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng F, Chen X, Liao Z, et al. A simplified and versatile system for the simultaneous expression of multiple siRNAs in mammalian cells using Gibson DNA Assembly. PLoS One. 2014;9(11):e113064. doi: 10.1371/journal.pone.0113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang E, Zhu G, Jiang W, et al. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res. 2012;27(7):1566–1575. doi: 10.1002/jbmr.1622. [DOI] [PubMed] [Google Scholar]
- 55.Toh WS, Liu H, Heng BC, Rufaihah AJ, Ye CP, Cao T. Combined effects of TGFbeta1 and BMP2 in serum-free chondrogenic differentiation of mesenchymal stem cells induced hyaline-like cartilage formation. Growth Factors. 2005;23(4):313–321. doi: 10.1080/08977190500252763. [DOI] [PubMed] [Google Scholar]
- 56.Estrada KD, Wang W, Retting KN, et al. Smad7 regulates terminal maturation of chondrocytes in the growth plate. Dev Biol. 2013;382(2):375–384. doi: 10.1016/j.ydbio.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Ionescu AM, Schwarz EM, et al. Smad6 is induced by BMP-2 and modulates chondrocyte differentiation. J Orthop Res. 2003;21(5):908–913. doi: 10.1016/S0736-0266(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 58.Li N, Lee WY, Lin SE, et al. Partial loss of Smad7 function impairs bone remodeling, osteogenesis and enhances osteoclastogenesis in mice. Bone. 2014;67:46–55. doi: 10.1016/j.bone.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y, Murao H, Yamamoto K, et al. Generation of transgenic mice for conditional overexpression of Sox9. J Bone Miner Metab. 2011;29(1):123–129. doi: 10.1007/s00774-010-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang HN, Park JS, Woo DG, et al. Chondrogenesis of mesenchymal stem cells and dedifferentiated chondrocytes by transfection with SOX Trio genes. Biomaterials. 2011;32(30):7695–7704. doi: 10.1016/j.biomaterials.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 61.Garza-Veloz I, Romero-Diaz VJ, Martinez-Fierro ML, et al. Analyses of chondrogenic induction of adipose mesenchymal stem cells by combined co-stimulation mediated by adenoviral gene transfer. Arthritis Res Ther. 2013;15(4):R80. doi: 10.1186/ar4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9(Suppl A):S69–75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 63.Ikeda T, Kamekura S, Mabuchi A, et al. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50(11):3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]