Abstract
The roles of non-muscle myosin II and cortical actin filaments in chromaffin granule exocytosis were studied by confocal fluorescence microscopy, amperometry, and cell-attached capacitance measurements. Fluorescence imaging indicated decreased mobility of granules near the plasma membrane following inhibition of myosin II function with Blebbistatin. Slower fusion pore expansion rates and longer fusion pore lifetimes were observed after inhibition of actin polymerization using Cytochalasin-D. Amperometric recordings revealed increased amperometric spike half-widths without change in quantal size after either myosin II inhibition or actin disruption. These results suggest that actin and myosin II facilitate release from individual chromaffin granules by accelerating dissociation of catecholamines from the intragranular matrix possibly through generation of mechanical forces.
Keywords: chromaffin cells, exocytosis, actin, myosin II, amperometry, capacitance, fluorescence microscopy, fusion pore
INTRODUCTION
Chromaffin cells of the adrenal gland are a widely used model system to study exocytosis (Jahn et al., 2003). The kinetics of catecholamine release from single chromaffin granules has been characterized in great detail using various approaches, such as amperometry (Wightman et al., 1991), capacitance measurements (Debus and Lindau, 2000) and patch-amperometry (Albillos et al., 1997). The small foot signal preceding amperometric spikes (Chow et al., 1992) is an indication of catecholamine release through the fusion pore formed between the vesicular lumen and the extracellular space, upon fusion of the secretory vesicle with the cell plasma membrane (Albillos et al., 1997).
Experimental evidence has been accumulated suggesting a role for the actin cytoskeleton in regulating neuroendocrine cell exocytosis (Malacombe et al., 2006). According to the current view, a meshwork of filamentous actin (F-actin) underneath the plasma membrane acts as a physical barrier to exocytosis (Aunis and Bader, 1988) that must be disassembled for vesicles from a reserve pool to enter the release-ready pool (Vitale et al., 1995). However, this model has been challenged by recent findings that suggest the participation of molecular motors, such as myosin Va, non-muscle myosin II and other actin binding proteins (Malacombe et al., 2006) in dynamic interactions with actin, supporting a more specific role for actin in the process of exocytosis. Biochemical studies have demonstrated association of myosin Va with chromaffin granules and reduction in secretion with anti-myosin V antibodies in permeabilized chromaffin cells has been reported (Rose et al., 2003). More recently, it was shown that pharmacological inhibition of myosin II and overexpression of an unphosphorylatable mutant of the regulatory light chain (RLC) of myosin II slowed down chromaffin granule movement as well as catecholamine release from single chromaffin vesicles (Neco et al., 2004; Neco et al., 2008).
However, the interaction between the actin cytoskeleton and the myosin molecular motors and how their interplay regulates secretion is unclear, specifically because myosin V but not myosin II has been found to interact with chromaffin granules (Rose et al., 2003). If the modulation of release kinetics by myosin II is mediated by interactions with actin filaments, then inhibiting actin polymerization would be expected to also affect individual secretory events. To investigate the roles of actin and myosin II in chromaffin granule mobility, fusion pore properties, and catecholamine release from single vesicles, we performed confocal fluorescence microscopy, amperometry and cell-attached capacitance recordings on single chromaffin cells following inhibition of either actin polymerization or the ATPase activity of myosin II.
MATERIALS AND METHODS
Cell preparation, reagents and solutions
Bovine chromaffin cells were prepared as previously described (Parsons et al., 1995). The buffered solution used for all the amperometric, capacitance and fluorescence measurements contained (in mM) 140 NaCl, 5 KCl, 5 CaCl2, 1 MgCl2, 10 HEPES/NaOH, 20 glucose (pH 7.3). The pipette solution used for the capacitance recordings contained (in mM) 50 NaCl, 100 TEA-Cl, 5 KCl, 5 CaCl2, 1 MgCl2, 10 HEPES/NaOH (pH 7.3). Ionomycin was purchased from Sigma and stock solution was prepared in ethanol. (-)-Blebbistatin, Cytochalasin-D, 1-(5-Iodonaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine hydrochloride (ML-7), and Latrunculin A were all purchased from Sigma and stock solutions were prepared in dimethylsulfoxide. Immediately prior to the beginning of an experimental session, stock solutions were diluted in the bath solution at a final concentration of 10 μM for Ionomycin, 4 μM for Cytochalasin-D, 10 μM for Blebbistatin, 3 μM for ML-7, and 2 μM for Latrunculin A. Chromaffin cells were incubated with the different inhibitors for 15 min at 37 C and 10% CO2 immediately prior to the recordings. A similar incubation was performed for control cells in order to take into account possible temperature effects on exocytotic activity (Pihel et al., 1996).
Quantification of cortical actin
Chromaffin cells treated with the different inhibitors were fixed with 3.7% formaldehyde for 10 min after 30 min incubation with the inhibitor. Cells were then permeabilized with 0.1% Triton X-100 for 5 min and actin filaments labeled with Alexa 568 phalloidin. Confocal microscopy was performed with a Leica TCS SP2 system with an acoustic optic-tunable filter and a 63× 0.9 NA water immersion objective. The density of cortical actin was quantified at the equatorial plane by integrating the total fluorescence intensity in an annular region containing the cell plasma membrane and dividing by the annulus area. The annular width was kept constant to 1.5 μm.
Vesicle tracking
Chromaffin granules were labeled with 3μM LysoTracker Green (Molecular Probes, Invitrogen Co.) for 5 min before imaging. Confocal microscopy was performed using the system described above with an optical slice thickness of ~0.9 μm at the interface between the glass surface and the cell plasma membrane. Images were acquired at a frame rate of 1.67 s-1 and the coordinates of individual vesicles were obtained by using the public domain program ImageJ. The vesicle tracking plug-in used was an implementation of an algorithm previously described (Sbalzarini and Koumoutsakos, 2005). Vesicles were automatically detected by the program after setting criteria for vesicle image size (circular spot of ≤500 nm diameter) and cut-off intensity (50% of the brightest particles detected). Vesicles were followed for several frames as long as they remained detected as a particle by the program. All tracks were overlaid with the original time series and visually inspected for accuracy. Only tracks longer than 10 frames were used for the analysis. After setting the selection criteria for vesicle size, cut-off intensity and trajectory length, 9-17 vesicles per cell were left for analysis from which the 9-10 brightest were chosen per cell to ensure that the tracking occurred for a similar number of vesicles per cell for all treatment groups. Mean squared displacements (MSD) were calculated as described (Qian et al., 1991) using a custom MATLAB (MathWorks) routine using the equation:.
where n and j are positive integers with n = 1, 2, ... (N-1). (x(jδt), y(jδt)) and (x(jδt + nδt), y(jδt + nδt) are the granule's coordinates at time jδt and jδt+nδt respectively (Manneville et al., 2003). The data was fitted to a simple diffusion model: MSD(nδt) = 4D nδt + c, where D is the diffusion coefficient and c is a constant that accounts for the limited accuracy of the experimental set-up (Manneville et al., 2003). All experiments were carried out in 35-mm Petri dishes with coverglass bottoms (0.16-0.19 mm; MatTek, Ashland, MA).
Amperometry
Amperometry was performed using custom made carbon fiber electrodes (CFEs) and a patch-clamp amplifier (EPC-8, HEKA-Elektronik, Germany). The current was low pass filtered at 500 Hz using the built-in analog low pass filter of the EPC-8 amplifier. The CFE was in touch with the cell surface, as verified visually by a slight deformation of the cell membrane. The CFE voltage was kept at +700mV versus a chlorinated silver reference electrode (Ag|AgCl). A glass pipette with ~2.5μm tip diameter containing 10 μM ionomycin solution was positioned ~40 μm away from the cell and a 3-sec 3.5 × 104 Pa puff was applied to the pipette using a pressure application system (PicoSpritzer II, Parker-Hannifin/General Valve, Fairfield, NJ, USA) to stimulate exocytosis. Amperometric recordings were performed for 10 min after stimulation and the data was digitized at 2 kHz rate by a 16-bit resolution NIDAQ board (BNC-2090, National Instruments, Austin, TX, USA). A digital notch filter at 60Hz (Igor Pro, WaveMetrics, Lake Oswego, OR, USA) was used to remove line frequency noise. Recordings were analyzed as previously described (Mosharov and Sulzer, 2005). Spikes with amplitude less than 10 pA, or half-width more than 300 ms, and overlapping spikes were excluded from the analysis. The 10 pA threshold was high enough for amperometric signals to be discerned from noise and low enough for the majority of amperometric spikes in all treatment groups to be included in the data analysis. The thresholds used for identifying foot signals were 1 pA amplitude and 5 ms duration.
Cell-attached capacitance measurements
High resolution capacitance measurements were performed in the cell-attached configuration as previously described (Debus and Lindau, 2000) using a HEKA EPC-7 amplifier and patch pipettes of nominal resistance between 1 and 2 MΩ. A dual lock-in amplifier (SR 830, Stanford Research Instruments, Sunnyvale, CA, USA) was used to obtain the complex admittance using a 50 mV-rms amplitude and 20 kHz frequency sine wave applied to the patch pipette. The lock-in amplifier outputs were digitized at 1 kHz rate by two 16-bit resolution channels of the NIDAQ board. Custom written software (Dernick et al., 2003) in Igor Pro converted the two orthogonal traces (real and imaginary part) into measurements of fusion pore conductance GP (units of nS) and vesicle capacitance CV (units of fF) as described (Debus and Lindau, 2000). From these recordings, vesicle size CV, fusion pore lifetime, fusion pore conductance, and fusion pore expansion rate were derived as described (Dernick et al., 2003). For this analysis, only exocytotic events with lifetime ≥15 ms were used (Dernick et al., 2003), since shorter events were heavily affected by the lock-in low-pass filters (τ=1 ms, 24 dB, which corresponds to 10 - 90% risetime of 5 ms) and their conductance properties are not reliably determined. The fusion pore initial expansion rate was calculated as the slope of a linear fit to the initial 15 ms segment of the conductance trace. The fusion pore lifetime was the time from fusion pore opening until the fusion pore conductance value exceeded 2 nS (Dernick et al., 2003).
Statistical analysis
All reported signal parameters, amperometric (quantal size, half-width, spike amplitude, mean foot signal amplitude and foot duration) and patch-capacitance (vesicle size, fusion pore initial and average conductance, fusion pore initial expansion rate and fusion pore lifetime), were statistically analyzed by taking the median values of the events from individual cells and subsequently averaging these values per treatment group. Therefore, data is represented as MEAN ± SEM, where n is the number of cells in each treatment group. Differences were considered to be statistically significant for p < 0.05 as assessed by Student's unpaired T-test for both the amperometric and patch-capacitance data. All experiments were performed at room temperature at day 1 after cell isolation. The data came from two and four different cell preparations for amperometry and capacitance respectively.
RESULTS
To investigate the roles of actin and non-muscle myosin II in exocytosis of chromaffin granules we used Cytochalasin-D and Latrunculin A, which inhibit actin polymerization, Blebbistatin, a specific inhibitor of non-muscle myosin II (Straight et al., 2003), and ML-7, an inhibitor of myosin light chain kinase (MLCK).
Blebbistatin treatment decreases vesicular motion
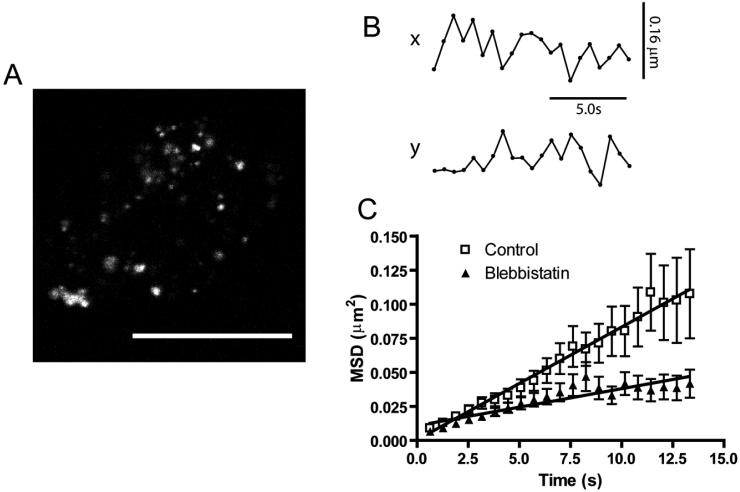
Myosin II and the actin cytoskeleton have been implicated in vesicular motion (Neco et al., 2004). We characterized vesicular movement in unstimulated cells using confocal microscopy focused on the actin rich cortical region of the cell (Fig. 1A). For this purpose, we tracked the motion of 94 vesicles from 9 untreated cells and 92 vesicles from 10 cells treated with Blebbistatin. The x- and y- coordinates of each vesicle were tracked in a series of images (Fig. 1B) and converted into mean squared displacement (MSD) for that particular vesicle. These were then averaged for all the cells per treatment group and plotted versus time (Fig. 1C). A linear fit to the data (Fig. 1C), revealed the apparent diffusion coefficient for the vesicles in each treatment group. The resulting apparent diffusion coefficients were 2.07 ± 0.06 × 10-3 μm2/s for control cells and 6.8 ± 0.8 × 10-4 μm2/s for Blebbistatin treated cells, thus ~3 fold lower in cells where the ATPase activity of myosin II was specifically inhibited compared to control cells.
Figure 1.
(A) Confocal micrograph of a representative cell used for vesicle tracking. Vesicles were labeled with Lysotracker green for 5 min and then imaged at 25°C without stimulation. Scale bar represents 20 μm. (B) Trace of typical xy coordinates followed by a single vesicle taken from control cells. (C) Plots of the two dimensional MSD calculated for control and Blebbistatin treated cells. Data is presented as mean ± SEM for 94 vesicles from 9 control cells, and 92 vesicles from 10 Blebbistatin treated cells.
Treatment with Cytochalasin-D or Blebbistatin did not affect intracellular calcium concentrations and protein kinase C distribution (Supplemental Figs. 1 and 2), indicating that the changes of vesicle mobility in Blebbistatin treated cells were specifically due to inhibition of myosin II and not a consequence of changes in intracellular calcium or protein kinase C activation, which may also affect vesicular motion, cortical actin distribution and exocytosis (Cuchillo-Ibanez et al., 2004).
Cytochalasin-D but not Blebbistatin affects cortical actin distribution
To test if the decreased mobility following inhibition of myosin II is a consequence of cortical actin destabilization, fluorescence microscopy was used to determine how Cytochalasin-D and Blebbistatin treatment affected the distribution of cortical actin fluorescence. As expected, Cytochalasin-D treated cells, showed disruption of cortical actin in contrast to Blebbistatin treated cells, which showed a similar distribution as control cells (Fig. 2A). Quantitative analysis (Fig. 2B) showed a 44% decrease in cortical actin fluorescence intensity (p < 0.001) in Cytochalasin-D treated cells, while Blebbistatin treated cells showed no significant difference (p > 0.35) when compared to control cells (Fig. 2C). These results indicate that the observed changes in vesicle mobility as well as the observed changes in release event properties (see below) are a direct consequence of myosin II inhibition in the absence of cortical actin disintegration. Calcium influx stimulated with ionomycin also produced a decrease in cortical actin as expected (Cuchillo-Ibanez et al., 2004), which was similar to that produced by Cytochalasin-D. Combined application of Cytochalasin-D and ionomycin did not produce a further decrease indicating that the loss of cortical actin reaches a limiting threshold (Supplemental Fig. 3). Blebbistatin treatment did not affect the distribution of myosin II (Supplemental Fig. 4), suggesting that the observed effects were not due to changes in the intracellular localization of myosin II. Interestingly, the peripheral localization was also retained in Cytochalasin-D treated cells indicating that the peripheral myosin II localization is not immediately lost upon disintegration of cortical actin.
Figure 2.
Effects of inhibition of actin polymerization and myosin II function on cortical actin distribution. (A) Confocal micrographs of chromaffin cells in control condition, and treated with 10 μM Blebbistatin or 4μM Cytochalasin-D. Cells were fixed after 30 min incubation with the inhibitor and stained for F-actin with Alexa 568 phalloidin. Scale bar represents 20 μm. (B) Schematic depicts the quantification of cortical actin. A circular region of interest inside the cell was subtracted from another region covering the entire cell. The annular width was kept equal to 1.5 mm for all cells. (C) Quantified fluorescence of Alexa 568 phalloidin labeled F-actin on the cortical region. Fluorescence values were normalized to the mean value for control cells. Data is presented as MEAN ± SEM from a total of 20 cells per group. Triple asterisks indicate p < 0.001 (Student's unpaired t-Test).
Inhibition of myosin II slows individual release events
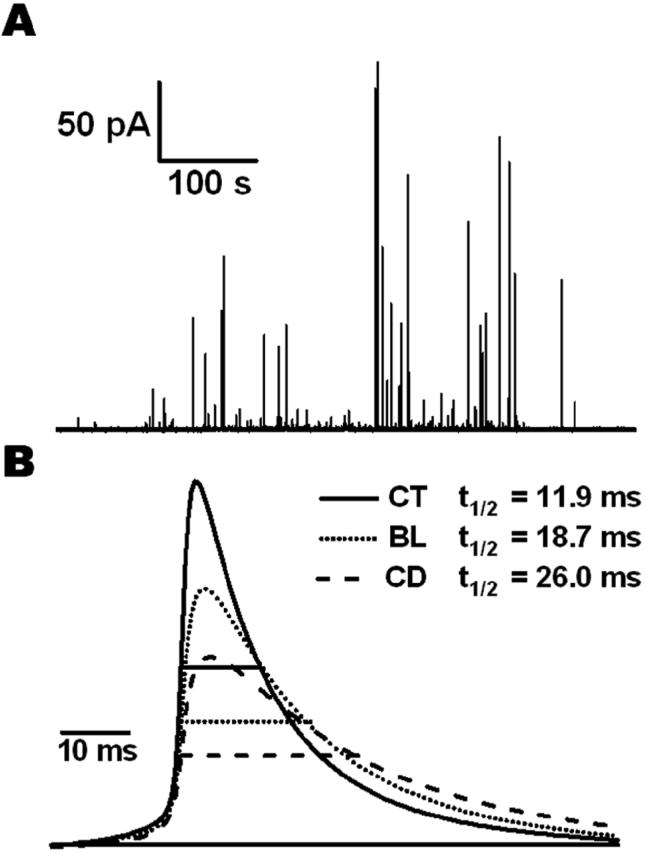
The kinetics of catecholamine release from single vesicles was determined by carbon fiber amperometry. Fig. 3A shows a typical recording from a chromaffin cell under control conditions. To characterize the average release kinetics an average amperometric spike shape was constructed (Fig. 3B). All amperometric signals detected from a single cell with amplitude >10 pA and half-width <300 ms were normalized to their peak amplitude, aligned in time at the point of their maximum slope (occurring shortly before the spike maximum) and averaged, providing the average spike shape for this cell. Subsequently, the average spikes from each cell in a treatment group were again averaged in the same way to obtain the average spike shapes for the different groups. Finally, the averaged spikes for the three groups were normalized such that they all had the same quantal size, consistent with the statistical analysis of integrated amperometric charge (see below). Both, Blebbistatin and Cytochalasin-D treated cells showed reduced spike amplitude with increased half-width.
Figure 3.
(A) A typical recording from an untreated chromaffin cell stimulated with ionomycin. (B) Spikes from each cell were normalized to their peak amplitude, aligned in time at the point of their maximum slope (occurring shortly before the spike maximum) and averaged, providing the average spike shape for this cell. The average spikes from each cell in a treatment group were again averaged in the same way to obtain the average spike shapes for control (CT, n = 19 cells, 786 spikes), Blebbistatin treated (BL, n = 18 cells, 633 spikes) and Cytochalasin-D treated (CD, n = 18 cells, 1229 spikes) cells. Last, the averaged spikes were normalized to the same quantal size. The half-widths of these averaged spikes were 11.9 ms for control, 18.7 ms for Blebbistatin, and 26.0 ms for Cytochalasin-D treated cells.
To determine the statistical significance of the changes in amperometric spike properties five parameters were determined for each spike: quantal size, amperometric spike half-width, peak amplitude, mean foot current amplitude and foot signal duration (Fig. 4A) (Mosharov and Sulzer, 2005). When the average spike half-width was determined for each cell and the mean for all cells in a treatment group was calculated, values of 12.7 ± 1.0 ms (control), 18.6 ± 1.2 ms (Blebbistatin), and 24.7 ± 2.2 ms (Cytochalasin-D) were obtained, in excellent agreement with the values from the averaged spikes (Fig. 3B).
Figure 4.
(A) A single amperometric spike along with the five parameters: quantal size Q (pC), half-width (ms), peak amplitude (pA), foot signal duration (ms), and mean foot current (pA). Averaged values for half-width (B), peak amplitude (C), quantal size (D), number of exocytotic events (E), mean foot current (F), and foot signal duration (G) for control (CT, n = 19 cells, 786 spikes), Blebbistatin (BL, n = 18 cells, 633 spikes), Cytochalasin-D (CD, n = 18 cells, 1229 spikes), ML-7 (ML7, n = 20 cells, 388 spikes), and Lat-A (LatA, n = 10 cells) expressed as percentage of control values (always taken to be 100%). Data is represented as MEAN ± SEM, where n is the number of cells. Differences between treatment groups were tested for statistical significance by Student's unpaired t-Test and are indicated by single (p < 0.05), double (p < 0.01) or triple (p < 0.001) asterisks.
A more robust method avoiding spurious artifacts due to outliers is to determine the median value for each spike parameter for each cell and subsequently calculate the mean of these median values for each treatment group (Fig. 4 B-G) (Mosharov and Sulzer, 2005). The half-widths determined with this method after normalizing to the control values were 100 ± 7.9 % (control, n = 19 cells), 153.5 ± 8.9 % (Blebbistatin, n = 18 cells), and 224.8 ± 22.8 % (Cytochalasin-D, n = 18 cells), confirming that the observed increase in spike half-width due to inhibition of myosin II function or due to inhibition of actin polymerization are highly significant (Fig. 4B). Consistent with these results, the inhibition of MLCK with the inhibitor ML-7 also increased the amperometric half-widths to a similar value as Blebbistatin (145.3 ± 12.2 %, p < 0.01, Fig. 4B). The increases in amperometric spike half-width by these treatments were accompanied by decreases in amperometric spike amplitude (Fig. 4C) with no significant changes in quantal size (Fig. 4D). To test whether the effects of Cytochalasin-D were specifically due to inhibition of actin polymerization, amperometric recordings were also performed in cells treated with Latrunculin A, which also hinders actin polymerization. Latrunculin A treatment produced an increase of amperometric spike half-width and decrease of spike peak amplitude without affecting quantal size (Fig. 4B-D), indistinguishable from the effects of Cytochalasin-D, indicating that these changes are specific consequences of actin depolymerization.
The number of exocytotic events recorded within 10 min after stimulation was unchanged when myosin II was inhibited (Fig. 4E). In contrast, in cells treated with ML-7 the number of events was significantly reduced to 33% of control (Fig. 4E, p < 0.01). This suggests that inhibition of MLCK by ML-7 may affect other molecules independent of myosin II, consistent with recent evidence (Xu et al., 2008), leading to the observed reduction in exocytotic events. However, in contrast to Blebbistatin and ML-7, Cytochalasin-D or Latrunculin A treatment increased the number of spikes by ~66% compared to control cells (Fig. 4E), in good agreement with the proposed role of actin as a barrier to exocytosis (Aunis and Bader, 1988; Rose et al., 2003).
Inhibition of actin polymerization but not myosin II affects the early fusion pore
The foot signal preceding single amperometric spikes (Chow et al., 1992) has attracted significant attention as it is directly related to the early fusion pore formed during chromaffin granule exocytosis (Albillos et al., 1997; Dernick et al., 2003; Gong et al., 2007). Neither inhibition of myosin II by Blebbistatin or ML-7 nor inhibition of actin polymerization by Cytochalasin-D and Latrunculin A had an effect on the mean foot current amplitude (Fig. 4F), suggesting that neither myosin II nor actin affect the structure of the early fusion pore. In contrast, the average foot signal duration was significantly increased (Fig. 4G) by ~65% in Cytochalasin-D (p<0.0001) and Latrunculin A (p<0.05) treated cells, but was unchanged by inhibition of myosin II with Blebbistatin or ML-7 (Fig. 4G). Foot signal duration could be reliably determined only for foot signals with duration ≥5 ms and amplitude ≥1 pA. The percentage of amperometric spikes that had a foot signal in this range was similar for control and Blebbistatin treated cells (33% and 35% respectively), but was increased to 45% for Cytochalasin-D treated cells, consistent with the overall increase in foot duration.
Alteration of early fusion pore properties by Cytochalasin-D
Time-resolved cell attached patch clamp capacitance measurements provide a more direct assessment of individual fusion pore properties. The data analysis (Fig. 5A) reveals the capacitance CV of the fused vesicle, the initial and average fusion pore conductance, the fusion pore lifetime and the fusion pore expansion rate (Lindau, 1991; Debus and Lindau, 2000). Vesicle capacitance (Fig. 5B), as well as initial and average fusion pore conductance (Fig. 5 C, D) were unchanged in cells treated with Blebbistatin or Cytochalasin-D. Thus, inhibiting myosin II function or actin polymerization has no effect on vesicle size, vesicular catecholamine concentration or early fusion pore structure. However, inhibiting actin polymerization by Cytochalasin-D prolonged significantly the fusion pore lifetime (Fig. 5E) and reduced the fusion pore expansion rate (Fig. 5F), explaining the observed increase in amperometric foot duration with unchanged foot current amplitude in amperometric recordings from Cytochalasin-D treated cells (Fig. 4 F & G). This data includes only detected fusion pores with lifetime ≥15 ms. The percentage of fusion pores with lifetime ≥15 ms was similar for control and Blebbistatin treated cells (21% and 30% respectively), but was increased to 51% for Cytochalasin-D treated cells.
Figure 5.
(A) The real (blue trace) and imaginary (red trace) parts of the complex admittance are converted into fusion pore conductance GP (green dots) and vesicle capacitance CV (black dots). The fusion pore initial and average conductance are depicted by the dashed horizontal black lines, while the fusion pore expansion rate is the slope of the linear fit to the initial 15 ms segment of the conductance trace (solid black line). The fusion pore lifetime is the time for the conductance to reach 2 nS from its initial value. (B) Vesicle step size, (C) fusion pore initial conductance, (D) fusion pore average conductance, (E) fusion pore lifetime and (F) fusion pore initial expansion rate for control (CT, n = 7 cells, 86 fusion pores), Blebbistatin (BL, n = 8 cells, 82 fusion pores), and Cytochalasin-D treated (CD, n = 8 cells, 78 fusion pores) cells. Data is represented as MEAN ± SEM, where n is the number of cells. Statistically significant differences (p < 0.05) are indicated by single asterisks.
Distribution of foot signal durations and fusion pore lifetimes
To better characterize the fusion pore kinetics, we constructed survival curves for the detected amperometric foot signal durations (Fig. 6A) and fusion pore lifetimes (Fig. 6B) for control, Blebbistatin treated and Cytochalasin-D treated cells. The survival curves for Blebbistatin treated cells are very similar to those for control cells whereas increased foot duration and fusion pore lifetime is evident for Cytochalasin-D treated cells. Accordingly, single exponential fits provided similar time constants τ for foot duration and fusion pore lifetimes in control and Blebbistatin treated cells but about twice as long for Cytochalasin-D treated cells (Table 1). However, single exponential fits failed to reproduce the survival curves accurately, as is particularly evident in the logarithmic plots (Fig. 6 C & D). This indicates that the kinetics is not homogeneous but reflects an inhomogeneous population with a distribution of rate constants. A distribution of activation energies (or log(k)) leads to kinetics that is better described by a power law function (Austin et al., 1973; Austin et al., 1975) , where k is the rate constant corresponding to the peak of the distribution and n corresponds to the width of the distribution (small n indicates a broad distribution). The power law fits reproduced the data well (Fig. 6, dotted lines). Table 1 provides the parameters returned from the fitting procedure for each treatment group. Again, the parameters for Blebbistatin treated cells are very similar to those for control cells. For Cytochalasin D treated cells the main difference is a much smaller parameter n, which indicates a much broader distribution of rate constants, extending to much longer foot durations and fusion pore lifetimes when actin polymerization is inhibited.
Figure 6.
Survival curves for (A) foot signal duration and (B) fusion pore lifetime. Logarithmic plots of survival curves for (C) foot signal duration and (D) fusion pore lifetime. A single exponential (dashed lines) and a power law function (dotted lines) were fitted to the data. Black: control (n = 7 cells, 86 fusion pores), green: Blebbistatin (n = 8 cells, 82 fusion pores), red: Cytochalasin-D (n = 8 cells, 78 fusion pores).
Table 1.
Fit parameters returned for the single exponential (τ) and power law (1/k, n) fits to the foot signal duration (Amperometry) and fusion pore lifetime (Capacitance) survival curves of each treatment group.
| Amperometry | Capacitance | |||||
|---|---|---|---|---|---|---|
| fit parameters | τ (ms) | 1 / k(ms) | n | τ (ms) | 1 / k(ms) | n |
| control | 28.6 ± 0.4 | 16.4 ± 0.2 | 2.4 ± 0.1 | 13.8 ± 0.2 | 8.1 ± 0.7 | 4.7 ± 0.5 |
| Blebbistatin | 28.4 ± 0.4 | 18.1 ± 0.2 | 2.8 ± 0.1 | 16.5 ± 0.4 | 7.7 ± 0.8 | 3.2 ± 0.3 |
| Cytochalasin-D | 63.8 ± 1.1 | 29.5 ± 0.4 | 1.6 ± 0.0 | 29.5 ± 0.7 | 8.8 ± 0.8 | 1.8 ± 0.1 |
The fraction of amperometric spikes with detectable foot signal and of fusion pores measured by capacitance measurements with lifetime ≥15 ms was increased in Cytochalasin-D treated cells compared to control and Blebbistatin treated cells (Table 2). This is consistent with the prolonged foot signal duration (Fig. 4G) and the increased fusion pore lifetime in Cytochalasin-D treated cells (Fig. 5E), which should increase the fraction of foot signals or fusion pores longer than the detection limits of 5 ms and 15 ms, respectively. The fitted data sets included only foot signals with duration ≥5 ms, and fusion pores with lifetime ≥15 ms, since shorter durations were affected by the respective low pass filters used and could thus not be reliably quantified. Table 2 compares the fraction of amperometric spikes and fusion events that fulfilled these criteria with the fraction of events predicted by the power law fits. While the fractions of fusion pore lifetimes ≥15 ms are in rather good agreement with the predictions from the power law fits, the fractions of amperometric spikes with a detectable foot signal is much lower than the predictions of the power law fit. However, this is not unexpected since foot signals may escape detection not only because of short duration but also due to small amplitude. The mean foot current amplitude calculated for all detected foot signals (not averaged per cell) was 3.5 ± 2.8 pA (mean ± s.d.) for control cells and similar for drug-treated cells. Since the detection limit was 1 pA, a significant fraction of foot signals with duration >5 ms will not be detected due to small amplitude.
Table 2.
Comparison of percentage of detected amperometric foot signals and fusion pores with predictions from power law fits for each treatment group.
| amperometric spikes with detectable foot | Expected fraction of foot signals ≥5 ms from power law fits | fusion pores with lifetime ≥15ms | Expected fraction of fusion pore lifetimes ≥15 ms from power law fits | |
|---|---|---|---|---|
| Control | 33% | 75% | 21% | 23% |
| Blebbistatin | 35% | 77% | 30% | 24% |
| Cytochalasin-D | 45% | 85% | 51% | 32% |
DISCUSSION
Reduced vesicular motion following inhibition of myosin II activity
Inhibition of myosin II reduced chromaffin granule mobility, consistent with previous reports (Lang et al., 2000; Neco et al., 2004). In contrast to Cytochalasin-D treatment, inhibition of myosin II did not lead to reduction of cortical actin filaments, indicating that the role of myosin II in chromaffin vesicle motion near the cell surface is not mediated by disintegration of the actin-rich cortex. Although myosin motor function is highly regulated (Somlyo and Somlyo, 2003), myosin activity at resting calcium concentration appears to contribute to vesicle mobility.
Frequency of exocytotic events
Inhibition of actin polymerization by Cytochalasin-D or Latrunculin A led to a 66% increase in the number of exocytotic spikes consistent with the role of actin as a physical barrier to exocytosis (Aunis and Bader, 1988). Blebbistatin treatment of chromaffin cells, however, did not result in a change of the number of measured exocytotic spikes, consistent with the presence of normal cortical actin filaments in these cells. In contrast, the non-specific MLCK inhibitor ML-7 reduced the number of exocytotic events, suggesting that ML-7 inhibits exocytosis via a mechanism that may not be mediated by inhibition of non-muscle myosin II (Tokuoka and Goda, 2006).
Inhibition of myosin II function or actin polymerization slows catecholamine release during amperometric spike phase
Inhibition of myosin II increased the average amperometric spike half-width, consistent with experiments using chromaffin cells overexpressing an unphosphorylatable mutant of the myosin II RLC (Neco et al., 2004). Inhibition of actin polymerization by Cytochalasin-D broadened the amperometric spikes even more than Blebbistatin. Myosin II could thus exert its role via interaction with or independent of actin. It has been suggested that tension in the vesicle membrane drives fusion pore expansion (Monck et al., 1991) and myosin II and actin may contribute to increased membrane tension helping to expand the fusion pore. It has so far not been possible to measure directly the fusion pore conductance in chromaffin cells during the amperometric spike. However, fusion pore dynamics can be resolved for the early fusion pore that gives rise to the amperometric foot signal. If F-actin and myosin II accelerate fusion pore expansion, we would expect that this should be reflected in the dynamics of the early fusion pore.
Modulation of early fusion pore expansion by F-actin but not myosin II activity
Indeed, inhibition of actin polymerization resulted in prolonged fusion pore lifetimes indicated by increased amperometric foot signal durations and increased narrow fusion pore lifetimes determined by cell-attached capacitance measurements. The fusion pore expansion rate was reduced while the initial and average fusion pore conductance as well as the average foot signal amplitude were unchanged. We conclude that cortical actin does not determine the structure of the early fusion pore, but facilitates the process of fusion pore expansion. Survival curves constructed for amperometric foot signal durations and fusion pore lifetimes were well fitted with power laws as expected for processes that reflect distributed kinetics based on a distribution of activation energies (Austin et al., 1973; Austin et al., 1975; Lindau and Rüppel, 1983). Fusion pore expansion is modulated by many factors including Ca2+ concentration (Fernández-Chacón and Alvarez de Toledo, 1995; Hartmann and Lindau, 1995) and PKC (Scepek et al., 1998) such that a kinetic heterogeneity is not unexpected. Inhibition of actin polymerization broadened the kinetic distribution towards longer fusion pore lifetimes providing the first direct evidence that actin contributes to fusion pore expansion in chromaffin cells.
Inhibition of myosin II activity, on the other hand, altered neither the early fusion pore structure, nor the fusion pore expansion rate or the early fusion pore lifetime, suggesting that myosin II is not mediating the role of actin during the early fusion pore. In contrast to our results, the expansion of the early fusion pore was slower in chromaffin cells overexpressing an inactive form of myosin II RLC (Neco et al., 2008). One possible explanation for this apparent discrepancy would be that Blebbistatin inhibition of myosin II may be incomplete and that the residual myosin II activity in Blebbistatin treated cells is sufficient to maintain normal fusion pore expansion kinetics. However, alternative explanations are at least equally possible. In our experiments Blebbistatin treatment was performed for 15 minutes prior to the experiment. In contrast, cells overexpressing the inactive form of Myosin II RLC were used one or more days after infection. Blebbistatin inhibition thus reveals the immediate consequences of myosin II inhibition and presumably its direct function in the release event. On the other hand, overexpression experiments may in addition reveal longer term consequences. Clearly, vesicle mobility is affected by myosin II inhibition and the changes in early fusion pore expansion may reflect longer term consequences of myosin II inhibition such as changes in vesicle maturation, docking or priming. The two experimental approaches are thus not directly comparable and provide complementary information.
In spite of normal early fusion pore dynamics, amperometric spike half-width was significantly increased in Blebbistatin treated cells, suggesting that the increased amperometric spike half-width may not be due to slower fusion pore expansion. It was suggested that dissociation from the granular matrix is the major process determining amperometric spike half-width (Jankowski et al., 1993; Wightman et al., 2002). The amperometric spike time course shows no strong correlation with quantal size (Schroeder et al., 1996) as would be expected for a rate limiting fusion pore. The time course of release of different granular contents from Cytochalasin-D treated PC-12 cells was also not correlated with the size of the particular compound, as would be expected for fusion pore limited release (Felmy, 2007). These results suggest that association with and dissociation from the intragranular matrix determine the kinetics of release. Additional support for this view came from a recent study showing that release events from chromogranin A null mice exhibit reduced amperometric spike half-widths (Montesinos et al., 2008).
Possible mechanisms for F-actin and myosin II function in exocytosis
The relaxation of membrane tension exerted by actin filaments on the cell plasma membrane in Cytochalasin-D treated cells may be responsible for slower fusion pore expansion. In contrast, inhibition of myosin II had no detectable effect on early fusion pore expansion suggesting that actin mediates fusion pore expansion by its interactions with other proteins (Dillon and Goda, 2005; Cingolani and Goda, 2008).
Myosin II, however, contributes to accelerating release during the amperometric spike. How can interactions of the extra-granular actin and non-muscle myosin II modulate catecholamine release kinetics from chromaffin granules? Our results suggest that mechanical forces (tension) on the granules may promote dissociation from the matrix and thus expel catecholamines. It has been proposed that in Xenopus eggs, cortical granules are compressed by F-actin during exocytosis, contributing to the driving force for granules to secrete their contents (Sokac et al., 2003). Non-muscle myosin II may exert its mechanical function on chromaffin granules by its ability to bind and contract filamentous actin. Release from the matrix appears to be governed by a low effective diffusion coefficient within the matrix (Amatore et al., 1999). The change in amperometric spike width might be a consequence of a changed effective diffusion coefficient that could result from mechanical forces exerted on the matrix affecting its catecholamine binding interactions. Alternatively it could be a consequence of a changed rate at which the surface of the granule matrix is exposed to the extracellular medium (Amatore et al., 1999) or the size of the exposed matrix area during the rapid release phase giving rise to the amperometric spike. However, considering that the amperometric spike time course appears to be independent of vesicle size the latter mechanism would require that the rate at which the membrane surrounding the vesicle is unwrapped or the finally exposed area is increased for larger vesicles. In either case, the role of myosin II is likely to exert mechanical forces on the granule by matrix compression or by expelling the matrix more rapidly, thus facilitating release by exposing the whole granule core to the extracellular solution and accelerating dissociation from the granular matrix.
The interactions between the vesicles and the cortical actin cytoskeleton could be mediated by myosin V, which has been localized to chromaffin granules (Rose et al., 2003), providing a possible link between an actin-myosin II scaffold and the secretory granule. However, interactions of myosin II with chromaffin granules should not be ruled out. The interaction of the secretory granules with actin filaments appears to be mediated by localized adaptor molecules, such as N-Wasp and ARP2/3 (Gasman et al., 2004) or Rab27A and MyRip (Desnos et al., 2003). One possibility is that upon stimulation the actin cortex redistributes to allow granules to collapse (Doreian et al., 2008). However, residual polymerized actin at the immediate fusion site may persist due to localized accessory molecules allowing actin-regulating proteins, such as myosin II to exert control on granule fusion, consistent with the unchanged localization of myosin II in Cytochalasin D or ionomycin treated cells where cortical actin is dramatically reduced. It thus appears possible that myosin II may dynamically interact with actin and secretory granules via currently unidentified adaptor proteins.
Supplementary Material
Acknowledgements
We thank Owasco Meat Co., Moravia, NY for providing bovine adrenal glands, Joan Lenz for excellent technical assistance and Dr. R. Molloy for many helpful discussions and critically reading the manuscript. This work has been supported by NIH Grant R01-NS038200, NIH 2T32GM007469 and the Nanobiotechnology Center (a Nantional Science Foundation Science and Technology Center, agreement No. ECS-9876771).
REFERENCES
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Amatore C, Bouret Y, Midrier L. Time-resolved dynamics of the vesicle membrane during individual exocytotic secretion events, as extracted from amperometric monitoring of adrenaline exocytosis from chromaffin cells. Chemistry-a European Journal. 1999;5:2151–2162. [Google Scholar]
- Aunis D, Bader MF. The Cytoskeleton as a Barrier to Exocytosis in Secretory-Cells. Journal of Experimental Biology. 1988;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- Austin RH, Beeson KW, Eisenstein L, Frauenfelder H, Gunsalus IC. Dynamics of ligand binding to myoglobin. Biochemistry. 1975;14:5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Austin RH, Beeson K, Ieisenst L, Frauenfe H, Gunsalus IC, Marshall VP. Dynamics of Carbon-Monoxide Binding by Heme Proteins. Science. 1973;181:541–543. doi: 10.1126/science.181.4099.541. [DOI] [PubMed] [Google Scholar]
- Chow RH, Rüden Lv, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Cuchillo-Ibanez I, Lejen T, Albillos A, Rose SD, Olivares R, Villarroya M, Garcia AG, Trifaro JM. Mitochondrial calcium sequestration and protein kinase C cooperate in the regulation of cortical F-actin disassembly and secretion in bovine chromaffin cells. J Physiol. 2004;560:63–76. doi: 10.1113/jphysiol.2004.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus K, Lindau M. Resolution of patch capacitance recordings and of fusion pore conductances in small vesicles. Biophysical Journal. 2000;78:2983–2997. doi: 10.1016/S0006-3495(00)76837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernick G, Alvarez de Toledo G, Lindau M. Exocytosis of single chromaffin granules in cell-free inside-out membrane patches. Nat Cell Biol. 2003;5:358–362. doi: 10.1038/ncb956. [DOI] [PubMed] [Google Scholar]
- Desnos C, Schonn JS, Huet S, Tran VS, El-Amraoui A, Raposo G, Fanget I, Chapuis C, Menasche G, de Saint Basile G, Petit C, Cribier S, Henry JP, Darchen F. Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J Cell Biol. 2003;163:559–570. doi: 10.1083/jcb.200302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- Doreian BW, Fulop TG, Smith CB. Myosin II activation and actin reorganization regulate the mode of quantal exocytosis in mouse adrenal chromaffin cells. J Neurosci. 2008;28:4470–4478. doi: 10.1523/JNEUROSCI.0008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmy F. Modulation of cargo release from dense core granules by size and actin network. Traffic. 2007;8:983–997. doi: 10.1111/j.1600-0854.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R, Alvarez de Toledo G. Cytosolic calcium facilitates release of secretory products after exocytotic vesicle fusion. FEBS Letters. 1995;363:221–225. doi: 10.1016/0014-5793(95)00319-5. [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell. 2004;15:520–531. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LW, de Toledo GA, Lindau M. Exocytotic catecholamine release is not associated with cation flux through channels in the vesicle membrane but Na(+) influx through the fusion pore. Nat Cell Biol. 2007;9:915–922. doi: 10.1038/ncb1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Lindau M. A novel Ca2+ -dependent step in exocytosis subsequent to vesicle fusion. FEBS Letters. 1995;363:217–220. doi: 10.1016/0014-5793(95)00318-4. [DOI] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jankowski JA, Schroeder TJ, Ciolkowski EL, Wightman RM. Temporal characteristics of quantal secretion of catecholamines from adrenal medullary cells. JBC. 1993;268:14694–14700. [PubMed] [Google Scholar]
- Lang T, Wacker I, Wunderlich I, Rohrbach A, Giese G, Soldati T, Almers W. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophysical Journal. 2000;78:2863–2877. doi: 10.1016/S0006-3495(00)76828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M. Time-resolved capacitance measurements: monitoring exocytosis in single cells. Quarterly Reviews of Biophysics. 1991;24:75–101. doi: 10.1017/s0033583500003279. [DOI] [PubMed] [Google Scholar]
- Lindau M, Rüppel H. Evidence for conformational substates of rhodopsin from kinetics of light-induced charge displacement. Photobiochemistry and Photobiophysics. 1983;5:219–228. [Google Scholar]
- Malacombe M, Bader MF, Gasman S. Exocytosis in neuroendocrine cells: new tasks for actin. Biochim Biophys Acta. 2006;1763:1175–1183. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Manneville JB, Etienne-Manneville S, Skehel P, Carter T, Ogden D, Ferenczi M. Interaction of the actin cytoskeleton with microtubules regulates secretory organelle movement near the plasma membrane in human endothelial cells. Journal of Cell Science. 2003;116:3927–3938. doi: 10.1242/jcs.00672. [DOI] [PubMed] [Google Scholar]
- Monck JR, Oberhauser AF, Alvarez de Toledo G, Fernandez JM. Is swelling of the secretory granule matrix the force that dilates the exocytotic fusion pore? Biophysical Journal. 1991;59:39–47. doi: 10.1016/S0006-3495(91)82196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MS, Machado JD, Camacho M, Diaz J, Morales YG, Alvarez de la Rosa D, Carmona E, Castaneyra A, Viveros OH, O'Connor DT, Mahata SK, Borges R. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci. 2008;28:3350–3358. doi: 10.1523/JNEUROSCI.5292-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Sulzer D. Analysis of exocytotic events recorded by amperometry. Nat Methods. 2005;2:651–658. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- Neco P, Giner D, Viniegra S, Borges R, Villarroel A, Gutierrez LM. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J Biol Chem. 2004;279:27450–27457. doi: 10.1074/jbc.M311462200. [DOI] [PubMed] [Google Scholar]
- Neco P, Fernandez-Peruchena C, Navas S, Gutierrez LM, de Toledo GA, Ales E. Myosin II contributes to fusion pore expansion during exocytosis. J Biol Chem. 2008;283:10949–10957. doi: 10.1074/jbc.M709058200. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Coorssen JR, Horstmann H, Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Pihel K, Travis ER, Borges R, Wightman RM. Exocytotic release from individual granules exhibits similar properties at mast and chromaffin cells. Biophys J. 1996;71:1633–1640. doi: 10.1016/S0006-3495(96)79368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Sheetz MP, Elson EL. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys J. 1991;60:910–921. doi: 10.1016/S0006-3495(91)82125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SD, Lejen T, Casaletti L, Larson RE, Pene TD, Trifaro JM. Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J Neurochem. 2003;85:287–298. doi: 10.1046/j.1471-4159.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- Sbalzarini IF, Koumoutsakos P. Feature point tracking and trajectory analysis for video imaging in cell biology. Journal of Structural Biology. 2005;151:182–195. doi: 10.1016/j.jsb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Scepek S, Coorssen JR, Lindau M. Fusion pore expansion in horse eosinophils is modulated by Ca2+ and protein kinase C via distinct mechanisms. European Molecular Biology Organization Journal. 1998;17:4340–4345. doi: 10.1093/emboj/17.15.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder TJ, Borges R, Finnegan JM, Pihel K, Amatore C, Wightman RM. Temporally resolved, independent stages of individual exocytotic secretion events. Biophys J. 1996;70:1061–1068. doi: 10.1016/S0006-3495(96)79652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003;5:727–732. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Tokuoka H, Goda Y. Myosin light chain kinase is not a regulator of synaptic vesicle trafficking during repetitive exocytosis in cultured hippocampal neurons. Journal of Neuroscience. 2006;26:11606–11614. doi: 10.1523/JNEUROSCI.3400-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale ML, Seward EP, Trifaro JM. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995;14:353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Troyer KP, Mundorf ML, Catahan R. The association of vesicular contents and its effects on release. Ann N Y Acad Sci. 2002;971:620–626. doi: 10.1111/j.1749-6632.2002.tb04540.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe DT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Gao XP, Ramchandran R, Zhao YY, Vogel SM, Malik AB. Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating beta2 integrins. Nat Immunol. 2008;9:880–886. doi: 10.1038/ni.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.