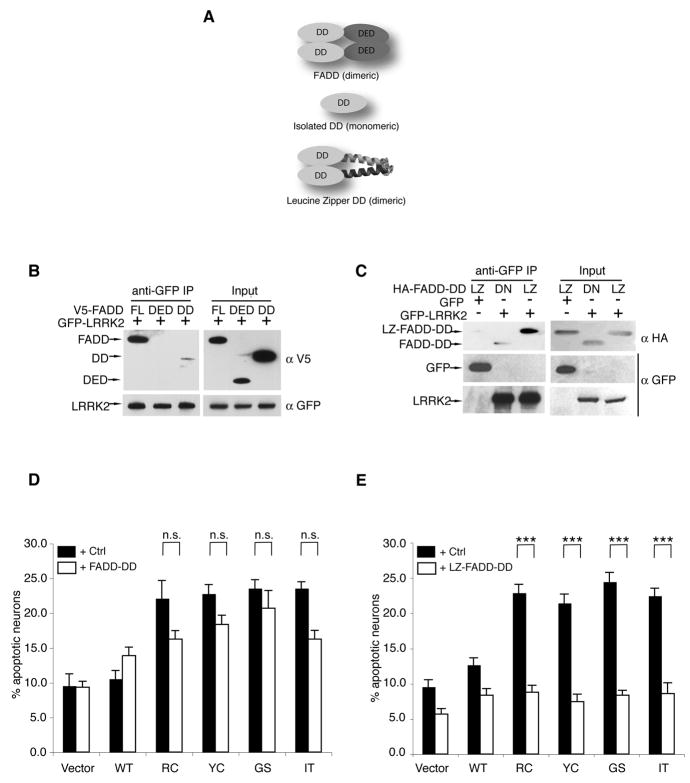
Figure 2. LRRK2-induced neuronal death requires FADD.
(A) A schematic depicts the domain structure of FADD, the isolated death domain (FADD-DD) and the leucine-zipper-DD (LZ-FADD-DD) in which the death domain is dimerized through the addition of a leucine zipper.
(B) FADD interacts with LRRK2 via its DD. GFP-LRRK2 was co-expressed with V5-tagged full-length FADD, FADD-DED, or FADD-DD in 293T cells and immunoprecipitated with anti-GFP. Co-purified FADD or FADD domains were detected by anti-V5 immunoblotting.
(C)LRRK2-FADD interaction is enhanced by dimerization of FADD-DD. The interaction between GFP-LRRK2 and monomeric (DD) or dimeric (LZ) FADD-DD was assessed by anti-HA following immunoprecipitation with anti-GFP in 293T cells.
(D)FADD-DD is a poor inhibitor of LRRK2 neurotoxicity. Mouse cortical neurons were transfected with LRRK2+lacZ (Ctrl) or LRRK2+FADD-DD. A GFP reporter was co-transfected in each case. Transfected neurons displaying apoptotic nuclear morphology were counted 48h following transfection using DAPI. Data are mean ± SEM from three individual experiments of triplicate coverslips (ns: non-significant, ANOVA with Tukey’s post-hoc test).
(E) Dimeric FADD-DD effectively blocks LRRK2 neurotoxicity. Mouse cortical neurons expressing LRRK+lacZ (Ctrl) or LRRK2+LZ-FADD-DD were assessed as in (D) (*** p<0.001).