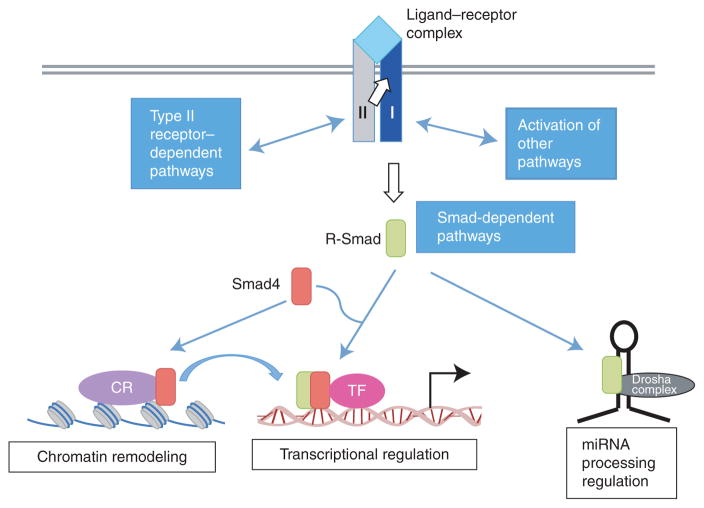
Figure 1.
Transforming growth factor β (TGF-β) receptors and signal transducers. TGF-β family ligands, shown in light blue, transmit signals by assembling a heterotetrameric receptor complex with two type I receptors, shown in dark blue, and two type II receptors, shown in gray. Upon ligand binding, signaling is transmitted by a cytoplasmic kinase domain of type I receptors by phosphorylating receptor-regulated Smad proteins (R-Smad proteins, green box). This is considered as the “Smad signaling pathway.” Additionally, the receptor complex can activate “non-Smad signaling pathways” through type II receptor– and type I receptor–interacting proteins. TGF-β and bone morphogenetic protein (BMP) receptors can also activate mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K) pathways. Activated R-Smad proteins form a complex with the common-Smad, Smad4 (co-Smad, shown in red box), and, as a complex, translocate to the nucleus, where they regulate transcription of target genes together with cofactors (pink circle). R-Smads also form a complex with chromatin remodeling proteins (CR, purple circle) that recognizes certain histone modifications and promotes formation of active chromatin, which is a prerequisite for transcriptional activation by R-Smad/co-Smad complexes. Additionally, R-Smad proteins can participate in microRNA (miRNA) processing by the Drosha microprocessor complex (black circle) for the biogenesis of a subset of primary transcripts of miRNA (pri-miRNAs).