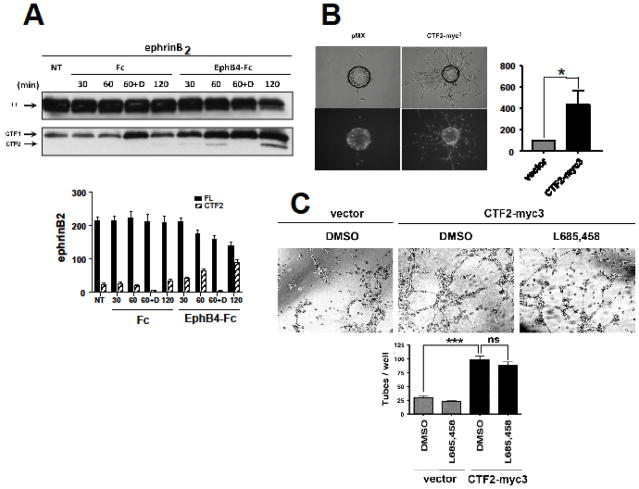
Fig. 1. EphB4-Fc stimulates efnB2 cleavage by γ-secretase producing peptide efnB2/CTF2 that regulates sprouting and tube formation.
(A). Upper: BAMECs transduced with efnB2 triple myc tagged (myc3) for detection purposes in pMX retroviral vector were stimulated with 2μg/ml pre-clustered Fc or EphB4-Fc for the indicated time points in the presence or absence of the γ-secretase inhibitor DAPT (D; 1μM). All samples were treated with the proteasomal inhibitor lactacystin (10μM). The extracts were IPed with a rabbit polyclonal antibody against c-myc to isolate exogenous myc-tagged efnB2. Cell extracts were immunoblotted with a rabbit polyclonal antibody against the C-terminus of efnB. Arrows indicate full-length efnB2-myc3 (FL) as well as the fragments produced by metalloproteinase and PS1/γ-secretase cleavage of efnB2-myc3 (CTF1 and CTF2, respectively). Lower: Quantification of full length efnB2 and efnB2/CTF2. N=3.
(B). BAMECs were transduced with pMX alone or vector expressing the cytoplasmic domain of efnB2 triple myc tagged (CTF2-myc3). Cells were grown on collagen-coated Cytodex® microcarrier beads, transferred to fibrinogen solution, and solidified with thrombin to form fibrin gels. Upper panel: Phase-contrast image of BAMEC cells sprouting from a microcarrier bead. Lower panel: Hoechst nuclear staining shows the number of endothelial cells that make up individual sprouts. The percentage of sprouts exceeding the diameter of the bead (~175μm) relative to control vector (pMX) was determined. Wilkoxon matched-pairs signed-rank (n=6, *p < 0.05, error bars = S.E.M.).
(C). BAMECs transduced with pMX alone or vector expressing CTF2-myc3 were seeded on a 24-well plate pre-coated with Matrigel™ Basement Membrane Matrix and left at 37°C/5% CO2 for 8–12hrs to spontaneously form tubes in the presence or absence of L685,458 (1μM). The plate was observed under a light microscope and the number of tubes formed was determined as a percentage relative to CTF2-myc3. Paired t-test (n=3, ***p < 0.001, error bars = S.E.M.).