Abstract
Human linkage and association studies suggest a gene(s) for nonsyndromic cleft lip with or without cleft palate (CL/P) on chromosome 4q31-q32 at or near the platelet derived growth factor-C (PDGF-C) locus. The mouse pdgfc−/− knockout demonstrates that PDGF-C is essential for palatogenesis. To evaluate the role of PDGF-C in human clefting, we performed sequence analysis and SNP genotyping using 1,048 multiplex CL/P families and 1,000 case-control samples from multiple geographic origins. No coding region mutations were identified, but a novel -986 C>T SNP (rs28999109) was significantly associated with CL/P (p=0.01) in cases from Chinese families yielding evidence of linkage to 4q31-q32. Significant or near significant association was also seen for this and several other PDGF-C SNPs in families from the US, Spain, India, Turkey, China, and Colombia, while no association was seen in families from the Philippines, Guatemala, and case-controls from Brazil. The -986T allele abolished six overlapping potential transcription regulatory motifs. Transfection assays of PDGF-C promoter reporter constructs demonstrate the -986T allele is associated with a significant decrease (up to 80%) of PDGF-C gene promoter activity. This functional polymorphism acting on a susceptible genetic background may represent a component of human CL/P etiology.
Keywords: PDGF-C, SNP, CL/P, promoter activity
Introduction
Isolated or nonsyndromic cleft lip with or without cleft palate (CL/P) is a common birth defect that affects ~ 1/700 newborns worldwide.1,2 Although the identification of genes for nonsyndromic CL/P is far from complete, genetic linkage and association studies in humans and animal models have identified at least 16 candidate loci for CL/P.1,3,4,5 Rare point mutations or significant association has been found between human nonsyndromic CL/P and missense mutations or polymorphic variants in several genes including PVRL1, IRF6, MSX1, RUNX2 and FGF signaling genes.1, 3, 4, 6 Only the IRF6 variant finding has been consistently replicated and recent evidence ascribes its affect to a point mutation in a TFAP2A binding site in an enhancer 10Kb upstream of the IRF6 promoter.6
Multiple lines of evidence support the existence of a human CL/P gene on distal 4q including significant associations with deletions of 4q31-qter,7,8 linkage with D4S192 9 and significant allelic association for CL/P with D4S192 in Caucasian10 and Chilean11 case-control studies The most significant result from multipoint linkage analysis of genome scan markers in Chinese multiplex CL/P families was found in 4q32 with the multipoint linkage peak at 158 Mb (fig. 1).12 The earlier positive linkage and association findings 9, 10,11 with D4S192 are not inconsistent with the Chinese results because earlier studies did not assess any markers distal to D4S192.
Figure 1.
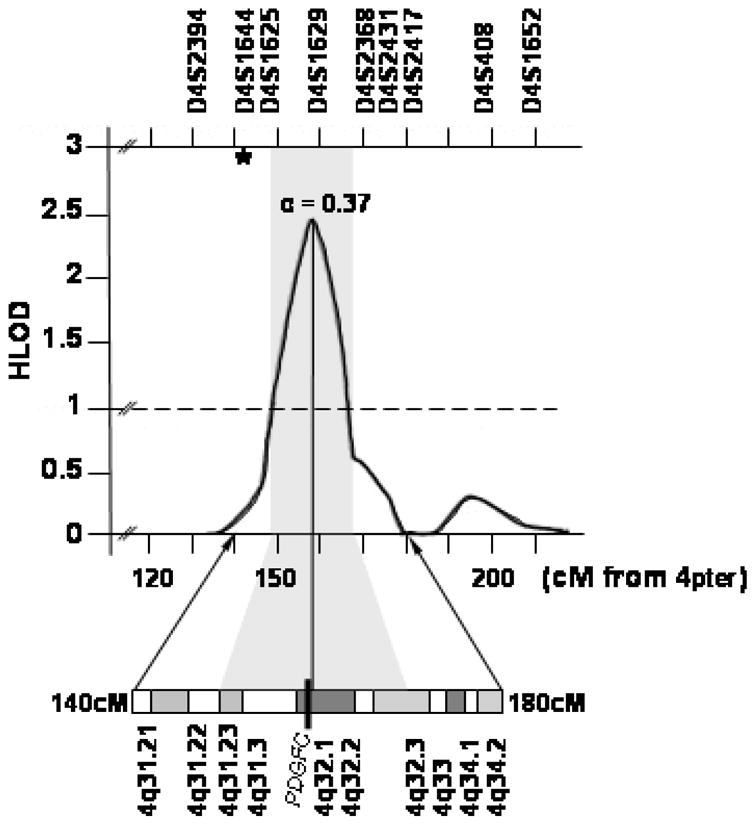
Multipoint linkage analysis results for chromosome 4q in multiplex Chinese families.12 Depicted are the multipoint heterogeneity LOD scores. α = the estimated proportion of linked families at the peak. The black bar across cytogenetic band 4q32.1 denotes the map position of the PDGF-C locus. The vertical grey-bar denotes the 1-LOD interval. Chromosomal marker locations and cytogenetic correlations are according to the Ensemble Database. The asterisk denotes the location of D4S192 (142 Mb from 4pter) reported to be significant in previous linkage 12 and association 10, 11 reports for CL/P in Caucasian and Chilean populations.
The human PDGF-C locus (157.89–158.12 Mb) maps to the linkage peak found in the Chinese families. Mouse knockout studies demonstrate PDGF-C is required for palatogenesis.13 While human studies support an etiologic role for several genes in CL/P etiology, (PVRL1, IRF6, and MSX1) 14,15,16, expression levels of the mouse homologues of these genes were unaltered in pdgfc−/− mutant embryos that develop clefts, suggesting their activity is not related to PDGF-C signaling in palatogenesis, so PDGF-C signaling is a new pathway in palatogenesis, independent of those previously identified.13
These findings led us to study the possible role of PDGF-C gene variants in human CL/P. Sequence analysis of the gene in Chinese CL/P cases and controls did not identify any coding region mutations in the PDGF-C gene. We did identify a novel SNP in the human PDGF-C gene promoter region that was associated with CL/P in Chinese families demonstrating linkage to chromosome 4q32.1. Evaluation of the role of this SNP on the transcriptional regulation of human PDGF-C gene expression indicates it may have a contributory role in some human CL/P cases. To evaluate the generality of these findings, multiple SNPs within PDGF-C were assessed for association with CL/P in 1,048 multiplex CL/P families from Europe, USA, Asia, Central and South America, and 1,000 case-control samples from Brazil.
Materials and Methods
Subjects
Study samples were drawn from 1,048 multiplex families, i.e. those with two or more individuals affected with nonsyndromic CL/P, from the following populations that have been described previously 17,18: USA and European Caucasians (Iowa; Texas; Pittsburgh; St. Louis; Madrid, Spain; and Turkey), Asia (Shanghai and Beijing, China; West Bengal, India; the Philippines), Central America (Guatemala), and South America (Colombia). An additional set of 500 cases and 500 controls from Brazil was also studied19. The families are summarized in Table 1. All studysubjects provided informed consent as approved by institutionalreview boards in boththe United States (University of Pittsburgh, University of Iowa) and each of the other countries involved.
Table 1.
Summary of families and individuals used in these analyses.
| INDIVIDUALS | |||||||
|---|---|---|---|---|---|---|---|
| POPULATION * | FAMILIES | Genotyped affected | Genotyped unaffected | Untyped Affected | Untyped Unaffected | Untyped Unknown | TOTAL |
| CAUCASIAN | |||||||
| Iowa | 117 | 126 | 220 | 4 | 9 | 0 | 359 |
| Pittsburgh | 98 | 132 | 292 | 13 | 89 | 1 | 527 |
| Madrid, Spain | 36 | 43 | 90 | 0 | 3 | 0 | 136 |
| Turkey | 29 | 32 | 55 | 6 | 195 | 0 | 288 |
| ASIA | |||||||
| India | 53 | 100 | 220 | 37 | 386 | 0 | 743 |
| Philippines | 242 | 664 | 1337 | 65 | 1366 | 0 | 3432 |
| China | 164 | 240 | 552 | 59 | 394 | 38 | 1283 |
| CENTRAL AMERICA | |||||||
| Guatemala | 77 | 82 | 310 | 11 | 111 | 0 | 514 |
| SOUTH AMERICA | |||||||
| Colombia | 232 | 349 | 577 | 15 | 368 | 0 | 1309 |
| TOTAL--Families | 1048 | 1768 | 3653 | 210 | 2920 | 40 | 8591 |
| Case-controls (Brazil) | 0 | 500 | 500 | 0 | 0 | 1000 | |
“Iowa” includes families from Iowa and Texas; “Pittsburgh” includes families from Pittsburgh, PA and St. Louis, MO; “China” includes families from Shanghai and Beijing, China.
One-hundred and eight affected individuals from the Shanghai, China study population were chosen for complete sequencing of the PDGF-C gene (one from each of the 104 families summarized in Table 1 plus an additional 4 cases that were the only participating individuals from their families). 26 cases came from “linked” families, i.e. families that individually had positive LOD scores with anonymous STRP markers in 4q31-q32, and 82 came from “unlinked” families.12 113 Chinese controls were also sequenced, 19 from the same Shanghai study population and 94 from the Coriell Han Chinese Human Variation panel (Coriell Cell Repository, Camden, NJ, URL http://ccr.coriell.org/nigms).
Families from Pittsburgh, St. Louis, Spain, India, Turkey, Guatemala, and the Philippines, and the Brazilian case-control samples were utilized for SNP genotyping of the novel SNP identified from sequencing these Chinese cases. All populations summarized in Table 1 except St. Louis, Guatemala and Brazil also had SNP genotyping available for 12 additional SNP’s within PDGF-C to investigate further the association between CL/P and PDGF-C (see Table 3).
Table 3.
Summary of FBAT analyses of PDGFC SNP’s in multiplex CL/P families, by population.
| SNP | MB location | Iowa | Pittsburgh | St. Louis | Spain | Turkey | India | Philippines | China | Colombia | Guatemala | Brazil | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs3815861 | T/C | 157.903698 | -- | 0.13 | -- | 0.03 | -- | -- | -- | -- | -- | -- | -- |
| rs1425486 | T/C | 157.903135 | 0.40 | 0.27 | -- | 0.22 | 0.20 | 0.67 | 0.48 | 0.26 | 0.01 | -- | -- |
| rs983473 | T/C | 157.914842 | 0.81 | 0.13 | -- | 0.02 | 0.28 | 0.54 | 0.49 | 0.37 | 0.03 | -- | -- |
| rs10517653 | T/G | 157.953440 | 0.38 | 0.16 | -- | 0.04 | 0.32 | 0.39 | 0.71 | 0.39 | 0.05 | -- | -- |
| rs2113992 | T/C | 157.977205 | 0.20 | 0.43 | -- | 0.43 | 0.14 | 0.81 | 0.87 | 0.50 | 0.02 | -- | -- |
| rs342318 | A/G | 158.008405 | 0.52 | 0.17 | -- | 0.76 | 0.32 | 0.70 | 0.92 | 0.33 | 0.08 | -- | -- |
| rs894588 | A/G | 158.036593 | 0.60 | 0.70 | -- | 0.61 | 0.18 | 0.84 | 0.43 | 0.63 | 0.83 | -- | -- |
| rs11728198 | A/G | 158.044847 | 0.75 | 0.70 | -- | 0.62 | 0.11 | 0.71 | 0.47 | 0.42 | 0.94 | -- | -- |
| rs6845322 | T/C | 158.103555 | 0.42 | 0.85 | -- | 0.61 | 0.34 | 0.43 | 0.57 | 0.77 | 0.78 | -- | -- |
| rs3733486 | G/A | 158.111884 | -- | -- | -- | -- | -- | -- | 0.42 | -- | -- | -- | -- |
| rs28999109 | C/T | 158.124911 | -- | 0.32 | 0.21 | 0.07 | 0.74 | 0.06 | 0.39 | *** | -- | 0.41 | 0.70 |
| rs1002091 | C/G | 158.112621 | -- | 0.19 | 0.04 | 0.35 | 0.09 | -- | 0.83 | 0.01 | -- | 0.50 | 0.24 |
| rs13133399 | T/G | 158.117234 | -- | 0.29 | -- | 0.34 | -- | -- | -- | -- | -- | -- | -- |
Results highlighted in yellow are statistically significant (p≤0.05), results highlighted in blue exhibit a trend near significance (0.05 < p ≤ 0.20).
see Table 2 for association results with this SNP in the Chinese families.
DNA sequence analysis of PDGF-C
Oligonucleotide primers were designed to amplify 1150 bases of the 5′ regulatory region and all 7 exons of the PDGF-C gene, including intron-exon boundaries (primer sequences available on request). Primers were designed using Oligo (Molecular Biology Insights, Cascade, CO, USA) and the PDGF-C genomic sequence contained in the chromosome 4 contig (NT_016354). All nucleotide numbering assumes the A of the ATG start codon as nucleotide 1. PCR amplification products were subjected to DNA sequencing using ABI dye-terminator chemistry and analyzed using an ABI 3100 DNA analyzer (Applied Biosystems, Foster City, CA, USA). Sequence results were compared to the reference sequences using the Sequencer Program (Applied Biosystems, Foster City, CA, USA). Identification of transcription factor binding sites was determined computationally using AliBaba2.20
Generation of human PDGF-C promoter reporter constructs
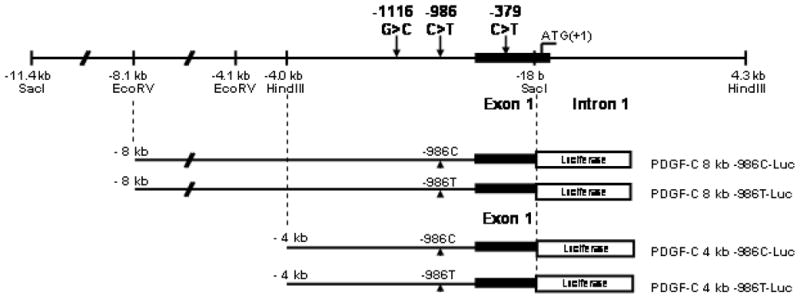
The human PDGF-C cDNA sequence (3 kb) was used as a probe for bioinformatics screening of the PDGF-C promoter DNA sequence using the NCBI database NT_016354.18. A 20 kb PDGF-C promoter sequence was identified and two PDGF-C containing bacterial artificial chromosome (BAC) clones were selected as described by Choi et al. 21 BAC clones (CTD-3161F17, CTD-2510M6, Open Biosystems, Huntsville, AL, USA) containing the PDGF-C promoter were subjected to further analysis. Amplified genomic DNA (130 kb to 170 kb insert) from the BAC clones was digested with restriction enzymes HindIII and SstI (New England Biolab, city, state, country) respectively. An 11.4 kb band digested with SstI and an 8.3 kb band digested with HindIII were eluted, subcloned (pBS KSII vector, Stratagene, La Jolla, CA, USA), and sequence verified. 8 kb human PDGF-C promoter DNA double-digested with EcoRV(partial digestion) and SacI and 4 kb human PDGF-C promoter DNA double-digested with EcoRV (from multiple cloning site of pBSKSII vector) and SacI (New England Biolab) were cloned into SmaI and SacI sites of pGL2 Enhnacer vector (Promega, Madison, WI, USA). Point mutagenesis substituting T for C at -986 in the human PDGF-C promoter was generated using the QuikChange® XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) according to the manufacturer’s protocol, followed by sequence verification. -986 C and -986T constructs were generated for both -1116C and -1116G backgrounds, so that 4 haplotypes were evaluated: -1116G-986C; -1116G-986T; -1116C-986C; and -1116C-986T (see fig 2). We generated two independent PDGF-C promoter reporter constructs (4kb and 8kb) to examine the -986 C>T SNP effects on its transcription activity.
Figure 2.
Genomic structure of human PDGF-C promoter and schematic diagrams of -986C and -986T allele human PDGF-C promoter Luciferase reporter constructs. 8 kb human PDGF-C promoter DNA double-digested with EcoRV (partial digestion) and SacI and 4 kb human PDGF-C promoter DNA double-digested with EcoRV (from the multiple cloning site of pBSKSII vector) and SacI were cloned into SmaI and SacI site of pGL2 enhancer reporter vector.
Measurement of -986C and -986T allele human PDGF-C promoter activity
-986C and -986T allele human PDGF-C promoter Luciferase reporter constructs were transfected into MC3T3, C2C12, Hela, and 293 cells (ATCC, Manassas, VA, USA) using a Lipofectamine Plus (Invitrogen, Carlsbad, CA, USA) kit as previously reported.22 Briefly, MC3T3, C2C12, Hela, and HEK293 cells (5X105/well) in 6-well plates were transfected with human PDGF-C promoter Luciferase reporter constructs (2 μg) and TK-Renilla Luciferase construct (0.2 μg). After 72 hours incubation in 5% CO2 at 37° C, clear cell lysates were harvested by microcentrifugation, and Firefly and Renilla Luciferase activity were measured using a dual-Luciferase reporter assay system (Promega, Madison, WI, USA) and the relative activity (ratio between Firefly and Renilla Luciferase activity) was calculated. Results are reported as the mean ± S.E. for three replicate samples using five independent -986C and -986T reporter constructs and were analyzed by Student’s t test. Results were considered significantly different for p < 0.05.
PDGF-C SNP Genotyping
To assess the generalizability of PDGF-C SNP associations in our study populations the novel -986 SNP (rs28999109 ) identified in the Chinese case-control study was genotyped in families from India, Turkey, Columbia, the Philippines, Pittsburgh, St. Louis, Guatemala, Spain, China, and case-controls from Brazil, utilizing TaqMan® chemistry with the Assay-On-Demand™ C_61773848_10 (Applied Biosystems, Foster City, CA, USA). Additional PDGF-C SNPs were analyzed to further investigate PDGF-C in CL/P (Table 3). As part of a larger CL/P fine-mapping project (ACL, MLM, JCM, LLF) DNA samples from the kindreds listed in Table 1 were genotyped for multiple SNPs within PDGF-C (see Table 3) by the Center for Inherited Disease Research (CIDR) utilizing the Illumina system (http://www.cidr.jhmi.edu/). Three additional SNPs were genotyped in some families using Assay-On-Demand™ probes, Applied Biosystems, Foster City, CA, USA (rs3733486: C_25803666_10; rs1002091: C_7427164_10; and rs13133399: C_9306502_10).
Statistical analysis
Preliminary analyses and case-control comparisons
Each SNP was assessed with PedCheck23 to test for inconsistencies due to non-paternity or other errors. Standard chi-square tests and Fisher exact tests were used to compare the SNP allele frequencies (from the sequencing studies) between the Chinese cases and controls, and between the Brazilian cases and controls. P-values of 0.05 or less were considered significant.
Allelic Association
Alleles at each PDGF-C SNP were tested for association with CL/P using the Family Based Association Test (FBAT). 24 Association was assessed for each SNP in each population, as well as the pooled data from all the populations, plus Caucasian, Asian, and Central/South American subsets. In addition to the individual SNP’s, association was also tested using the haplotype version of FBAT (HBAT) 24 in the individual populations and pooled subsets for sliding windows across the PDGF-C SNPs.
Results
Sequencing of the human PDGF-C gene
Direct DNA sequence analysis of the PDGF-C coding regions did not identify any coding region mutations in Chinese cases or controls, but three SNPS were identified. A known C>T SNP (rs3733486), was detected in the noncoding region of exon 1 (-379 from the ATG start codon), in one unaffected and two affected individuals. Two additional SNPs were identified in the 5′ regulatory domain of the PDGF-C gene: a known G>C substitution at -1116 (rs1002091), and a novel C>T substitution at -986 (Submitted to dbSNP; assigned rs28999109), see fig. 2. There was no significant difference in the frequency of the -1116 G>C variant between cases and controls (p = 0.21). Table 2 summarizes the frequency of the C>T SNP variant at -986 in CL/P cases from 4q31-q32 linked and 4q31-q32 unlinked Chinese families, and among Chinese controls. The frequency of the T allele in cases from linked Chinese families was significantly greater than in controls (case allele frequency= 0.15; control= 0.06; p= 0.01), but not for cases from unlinked families (frequency= 0.08; p= 0.34), while the frequency in all cases was borderline significant (frequency= 0.10; p= 0.09
Table 2.
Sequencing results for the -986 nucleotide position of the human PDGF-C gene promoter in 108 affected individuals from Chinese CL/P families and 113 Chinese controls.
| Genotype | ||||||
|---|---|---|---|---|---|---|
| N | C/C | C/T | T/T | χ2 (1 d.f.) | p-value | |
| CASES—from linked families a | 26 | 21 | 2 | 3 | 6.10 | 0.014 |
| CASES—from unlinked families a | 82 | 71 | 9 | 2 | 0.91 | 0.341 |
| CASES---TOTAL | 108 | 92 | 11 | 5 | 2.84 | 0.090 |
| CONTROLS—TOTAL | 113 | 102 | 9 | 2 | -- | -- |
Cases are from multiplex CL/P families from Shanghai, China. “Linked families” are families with evidence of linkage to anonymous STRP marker D4S1629 in 4q31-q32, HLODMAX is 158 Mb from 4pter.9
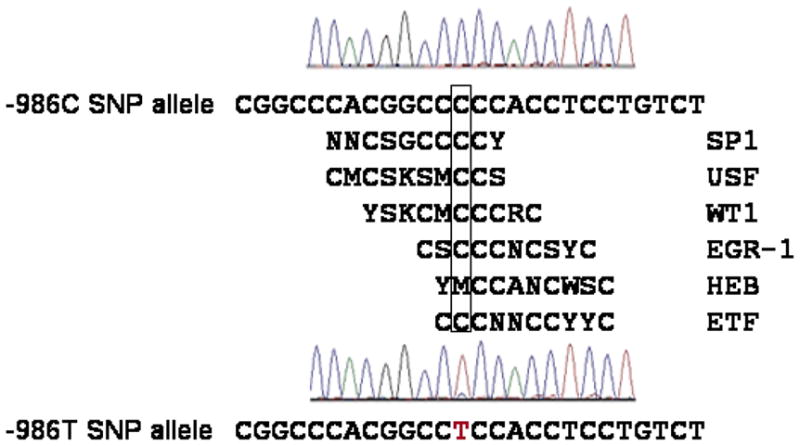
The presence of the wild-type cytosine nucleotide allele at -986 from the ATG start codon (-986C) is associated with 6 overlapping transcription factor binding consensus motifs including, EGR-1, Sp1, WT1, USF, HEB, and ETF (fig 3.). Substitution of thymine at the nucleotide 986 position (-986T) abolishes these cytosine-rich regulatory motifs.
Figure 3.
A C>T SNP (rs28999109) at -986 in the human PDGF-C promoter regulatory region abolishes consensus binding motifs for Sp1 (Specific protein 1); USF (upstream stimulatory factor), WT1 (Wilms tumor zinc finger protein-1), EGR-1 (early growth response factor-1), HEB (human B-HLH factor), and ETF (epidermal growth factor receptor (EGFR)-specific transcription factor) (M=A,C; S=C,G; K=G,T; Y=C,T; R=A,G; W=A,T; N=A,C,G,T).
Effects of -986C and -986T SNPs in the human PDGF-C promoter on the transcriptional regulation of PDGF-C gene
To determine the effects of the -986C/T alleles on PDGF-C transcriptional regulation, -986C PDGF-C and -986T PDGF-C allele Luciferase reporter constructs were co-transfected with the TK-Renilla Luciferase construct into MC3T3, C2C12, and HEK-293 cell lines. The relative activity of 4 kb -986T allele PDGF-C promoter was significantly decreased in MC3T3 cell lines (about 80%) (fig. 4a) and in C2C12 (fig. 4c) and HEK293 (fig. 4e) cell lines (about 50%) compared to those of 4 kb -986C PDGF-C constructs.
Figure 4.
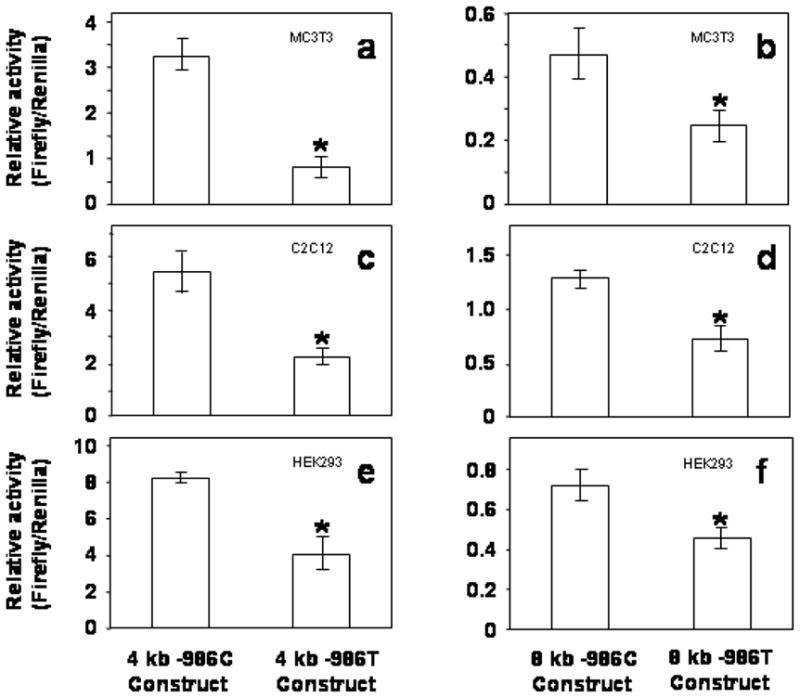
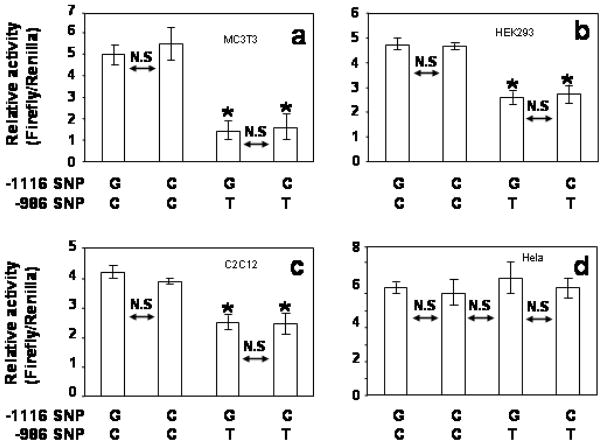
Presence of the -986T SNP (rs28999109) in the human PDGF-C promoter reduces promoter activity. Human PDGF-C Luciferase reporter constructs (2ug) containing either the -986C or the -986T allele were co-transfected with the TK-Renilla Luciferase construct (0.2ug) into mouse osteoblastic precursor cells (MC3T3) (a, b), mouse myoblastic cells (C2C12) (c, d), and human embryonic kidney epithelial cells (HEK293) (e, f) using Lipofectamine Plus kits. Relative activities of the 4 kb -986T allele PDGF-C promoter construct are significantly decreased (> 50%) compared to that of the 4 kb -986C allele PDGF-C promoter construct in all three cell lines (a, c, e). Relative activities of 8 kb -986T allele PDGF-C promoter construct are also significantly decreased (> 40%) compared to those of 8 kb -986C PDGF-C promoter construct in these cell lines (b, d, and f).
Moreover, relative activities of the 8 kb -986T PDGF-C promoter constructs are also significantly decreased compared to those of the 8 kb -986C allele PDGF-C promoter (fig. 4b, 4d, 4f) in all three cell lines. Although transcriptional activities of the 4 kb PDGF-C 5′ promoter constructs are more than 5 fold greater than all activities associated with the 8 kb promoter constructs, transcriptional activities of all constructs containing the -986T allele were consistently decreased 40% or more compared to -986C constructs (fig. 4), suggesting that this region (from −8 kb to −4 kb) of the human PDGF-C promoter may contain tissue specific negative regulatory domain(s). Although there was no statistically significant association with affection status for either allele at -1116, given its close proximity to the -986 SNP within the regulatory region, we measured PDGF-C gene promoter activity containing all 4 possible haplotypes of 4 kb reporter constructs (-1116G -986C; -1116G -986T; -1116C -986C; and -1116C -986T) to evaluate the effect of the -1116 G>C SNP on the transcriptional regulation of PDGF-C gene expression. As shown in figure 4, -986T allele PDGF-C reporter constructs significantly decreased promoter activities compared to those of -986C constructs regardless of -1116G or -1116C in MC3T3 (fig. 5a), HEK-293 (fig. 5b), and C2C12 (fig. 5c) cell lines. In contrast, -986T allele PDGF-C reporter constructs did not decrease the promoter activity of human PDGF-C gene in Hela cell line suggesting that Hela cells may not have transcriptional regulatory machinery for the human PDGF-C gene (fig. 5d).
Figure 5.
Effects of -1116 G>C SNP on PDGF-C promoter activity. Presence of the -986T SNP in the human PDGF-C promoter reduces promoter activity regardless of genotype at the -1116 position. Four haplotypes (-1116G -986C; -1116C -986C; -1116G -986T; -1116C -986T) were generated using the 4kb human PDGF-C promoter reporter construct. The presence of the -986T SNP resulted in a significant decrease the PDGF-C promoter activity in MC3T3 cell line (a), 293 cell line (b), and C2C12 cell line (c) regardless of the -1116 genotype. However, the -986 T allele did not affect PDGF-C promoter activity in Hela cell line (d).
Allelic association analyses of PDGF-C SNP’s and CL/P
The results of the FBAT analyses of multiple PDGF-C SNP’s are summarized in Table 3. The novel -986 PDGF-C SNP (rs28999109) was nearly significantly associated with CL/P in the West Bengal, India families (p-value=0.06), and Spanish families (p-value=0.07) but not in the Turkish, Pittsburgh, St. Louis, Guatemala, nor Filippino families (p-values>0.21). Other PDGF-C SNPs were also significantly associated with CL/P in some of the populations, notably within the Colombian, St. Louis, Spanish and Chinese samples (see Table 3). Further, results showed a trend towards significance in additional populations (Iowa, Pittsburgh, Turkey). The results from the Brazilian case-control sample were not significant and are not shown in detail. Interestingly, case-control analyses from the Chinese families showed significant association with the -986 SNP, and family-based analyses also showed association with an adjacent SNP (rs10020901)_in China (see Table 3, p-value = 0.02), St. Louis (p-value=0.04) and near significant association in PIttsubrgh and Turkey. The pooled FBAT analyses showed a trend towards significance for some of the SNPs in the TOTAL and Caucasian subsets. Haplotype analyses also had population-specific patterns in significance. Table 4 shows the most notable results: a three-SNP haplotype in the Caucasians (comprising SNPs rs983473, rs10517653, rs10517653) was significantly associated with clefting (p-value 0.04), as was a two-SNP haplotype in the Chinese (the -986 SNP rs28999109, and rs1002091; p-value= 0.04)
Table 4.
Sliding window haplotype association results for Caucasian and Chinese subsets.
| 4A. Three-SNP windows in Caucasians | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Caucasians | |||||||||||
| rs3815861 | rs1425486 | rs983473 | rs10517653 | rs2113992 | rs342318 | rs894588 | rs11728198 | rs6845322 | rs28999109 | rs1002091 | rs13133399 |
| 0.099775 ** | |||||||||||
| 0.183495 | |||||||||||
| 0.036706 * | |||||||||||
| 0.294555 | |||||||||||
| 0.345681 | |||||||||||
| 0.734077 | |||||||||||
| 0.297771 | |||||||||||
| 0.693876 | |||||||||||
| 0.524789 | |||||||||||
| 0.632203 | |||||||||||
| 4B. Two-SNP windows in the Chinese | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chinese | |||||||||||
| rs1425486 | rs983473 | rs10517653 | rs2113992 | rs342318 | rs894588 | rs11728198 | rs6845322 | rs28999109 | rs1002091 | ||
| 0.522842 | |||||||||||
| 0.629394 | |||||||||||
| 0.683704 | |||||||||||
| 0.577984 | |||||||||||
| 0.585417 | |||||||||||
| 0.571157 | |||||||||||
| 0.840722 | |||||||||||
| 0.697151 | |||||||||||
| 0.036375 * | |||||||||||
statistically significant (p<0.05)
borderline significant (p<0.10),
Discussion
Animal studies demonstrate that PDGF signaling is important in palatal development, with a specific role for PDGF-C.13, 25 Evidence from genetic linkage, association and cytogenetic deletions provide support for a human CL/P locus in the chromosome 4q31-ter region containing the PDGF-C locus.7–12 These observations led us to evaluate the PDGF-C gene for genetic variants that may be etiologic for human CL/P. Sequence analysis identified a novel SNP polymorphism (rs28999109) in the proximal domain of the PDGF-C gene in Chinese CL/P families demonstrating maximum linkage to the PDGF-C locus.12 The presence of the less frequent rs288999109 T allele disrupts a highly conserved cytosine-rich regulatory motif in the 5′ proximal regulatory region of PDGF-C that has consensus sequence for several DNA binding transcription factors that modulate PDGF expression.26,27 Reporter constructs containing the T allele demonstrate significantly decreased promoter transcription compared to constructs containing the more common C allele (p < .05). Decreased PDGF-C expression has been correlated with orofacial clefting in several mouse models.13, 28, 29, 30 Our finding of a positive association between the rarer rs28999109 T allele in the PDGF-C 5′ regulatory region and CL/P in a subset of Chinese families is consistent with a plausible etiologic mechanism for a complex trait such as CL/P. Further evidence for a role of PDGF-C in CL/P is provided by our results showing statistically significant association between additional PDGF-C SNPs and CL/P in study samples from St. Louis, Spain and Colombia, and a trend near significance in other study populations (Iowa, Pittsburgh, Turkey). Larger study samples will be necessary to confirm the trends towards significance. The results of this study imply population-specific associations with PDGF-C in that one of the largest study samples (the Philippines) showed no evidence of association. Interestingly two of the populations showing associations with the novel -986 SNP (China and India) were both of Asian origin but the remaining Asian population (the Philippines) did not show significant association with any PDGF-C SNPs. The populations that showed significant or near-significant association with other PDGF-C SNPs were all Caucasian (Spain, Iowa, Pittsburgh, and Turkey) or mixed Caucasian/Native American (Colombia). While results in data pooled across populations was either borderline or not significant, this is not unexpected given the population-specific patterns in the results, which may also contribute to the well-known differences in the epidemiological characteristics of nonsyndromic CL/P.1,2 We estimated the population attributable risk for the -986 allele in the Chinese to range from 4.2% to 6.4%; therefore one possible explanation for the population-specific differences in the association results may be differences in proportion of risk.
PDGFs play distinct roles at successive stages of mammalian organogenesis.31 PDGF ligands regulate biological processes by binding to and activating PDGF receptors (PDGFR). This activates the tyrosine kinase domain contained within the intracellular portion of PDGFRs and initiates intracellular signaling events (Erk/MAPK and Akt/PKB) that trigger cellular responses such as proliferation, migration, contraction and cell survival, essential for numerous biological processes.32 This PDGF signaling specificity is mediated through the activity of multiple immediate early genes (IEGs).33 PDGF-dependent tissues include the vasculature, kidney, neural crest-driven skeleton, and thoracic skeleton as well as the branchial arches and craniofacial mesenchyme. Identification and validation of PDGF transcriptional targets have been determined, and the specificity of downstream function is dependent upon PDGF receptor/ligand activation of specific IEGs.33, 34
PDGF-C can bind and activate PDGFR -αα and –αβ. PDGFRα is required for neural crest cell development and normal craniofacial development.35 PDGF-C has been characterized as a key component of PDGFR-α signaling by biochemical analyses 36 and in vivo gene-targeting.13 Nonsyndromic cleft palate derives from an embryopathy with consequent failure of the palatal shelf fusion.29 PDGFR-α and PDGF-C are key regulators for embryonic and postnatal development, and are required for normal palatogenesis.13, 25, 29, 30, 33, 35, 37 Animal models demonstrate that disruption of pdgfr-α signaling through a variety of etiologies including genetic mutation of pdgfr-α 35, microRNA suppression of pdgfr-α translation38, genetic mutation of PDGF-C ligand13, 28, suppression of PDGF-C transcription29 and suppression of PDGF-C protein expression by retinoic acid29, 30, are all associated with disruption of palatogenesis and the presence of orofacial clefting. While mutations and knockout studies of pdgfr-α and PDGF-C are etiologic for defective palatogenesis and orofacial clefting, knockout studies in mice indicate that other genes etiologic for CL/P (PVRL1, IRF6, MSX1) are not altered, indicating that pdgf-c signaling is a new and independent mechanism that regulates palatogenesis.1, 13
The proximal domain of PDGF gene promoters can directly modulate gene expression.39 PDGF-A and PDGF-C share common gene regulatory mechanisms, and their expression is controlled by the zinc finger transcription factors Sp1 and EGR-1, which have affinity for overlapping nucleotide recognition elements.26, 27, 40 Additionally, through its ability to repress expression of EGR-1, WT1 may function as a component in a transcription factor complex that regulates PDGF-C expression.41 The presence of the more common cytosine nucleotide in rs28999109 at nucleotide position -986 from the ATG start codon in the PDGF-C gene preserves 6 overlapping transcription factor binding consensus motifs (including Sp1, EGR-1 and WT1) within a highly conserved 500 bp interval. Substitution of a thymidine nucleotide at this position in rs28999109 abolishes the consensus sequence for multiple DNA binding transcription factors including Sp1, EGR-1 and WT1. The transcriptional activities of PDGF-C promoter reporter constructs containing the rs28999109 T allele were consistently decreased more than 40% compared to constructs containing the C allele in mesenchymal C2C12 cells and in osteoprogenic MC3T3 cells differentiated from mesenchyme, suggesting that disruption of the consensus sequence may reduce PDGF-C expression in these cells. Decreased PDGF-C transcription secondary to the less frequent rs28999109 T allele may act on a susceptible genetic background to impair PDGFRα-PDGF-C signaling, increasing susceptibility to CL/P. Genetic variants in downstream IEGs critical to craniofacial and palatal development could contribute to susceptibility as demonstrated in the mouse model.33
Mutations in PDGFR-α 35, as well as mutations of their PDGF ligands13, 28, including PDGF-C, disrupt craniofacial development and are associated with craniofacial clefting and defective palatogenesis. PDGF signaling specificity is mediated through immediate early genes.33 Mutations of specific immediate response genes (Arid5b, Tiparp, Sgpl1, BCo55757, Axud1, Mzf6d and Schip1), which are the downstream gene targets controlled by the PDGF pathway33, 34, are also associated with anomalies of craniofacial development. The associated phenotypes are consistent with the facial clefting seen in pdgfrα−/− mice, but are less severe and less penetrant. Additionally, while pdgfrα−/− mice show severe skeletal defects, loss of one copy of pdgfrα (pdgfrα−/+) increased the severity of skeletal defects (including palatal clefting) in many lines with IEG mutations (Arid5b, Tiparp, BCo55757, BC058969 and Schip1), and created skeletal malformations in one IEG knockout line that had no previous skeletal defects (Plekha1).33 These studies demonstrate that mice with mutations in the primary gene targets of PDGF signaling, the immediate early genes (IEGs), show phenotypes in the same structures and cell types as seen in PDGF receptor mutants, supporting the notion that target genes control specific processes downstream of individual receptor tyrosine kinases (RTKs). IEGs are specific for individual RTKs and responsible for particular downstream functions. Additionally, defective craniofacial development was seen as a result of dosage-sensitive genetic interactions with PDGF signaling genes and their downstream targets (IEGs), suggesting that these genes work collectively to implement PDGF function in development.33
Nonsyndromic cleft palate derives from an embryopathy with consequent failure of palatal shelf fusion.29 The etiology of this condition is complex, and multiple genes and environmental factors are likely involved.3, 4 As a key component of the PDGFR-α signaling pathway, PDGF-C is an important regulator of cell proliferation, survival, migration as well as deposition and maintenance of extracellular matrix.32 Inactivation of PDGF-C or reduced PDGF-C expression in genetic (Pdgf-c −/−) or teratogen (Retinoic acid) models perturbs regulation of MMPs and TIFs in palatal mesenchyme during branchial arch development.30, 42, 43 PDGF-C is a potent mitogen, and promotes proliferation of mouse embryonic palatal mesenchymal (MEPM) cells, and is required for branchial arch morphogenesis.29 PDGF-C expression is also influenced by FGF signaling and small ubiquitin-like modifier modification (SUMO).44 As alterations of FGF signaling are etiologic for some forms of orofacial clefting45, 46, SUMO and PDGF-C may also interact with environmental risk factors to influence CL/P susceptibility.
The findings of this study demonstrate the importance of applying both animal and human model approaches in the search for developmental and etiological mechanisms underlying complex traits. Just as mutations in different genes in the PDGF signaling pathway work collectively to implement PDGF function in development33, mutations and functionally significant genetic polymorphisms in different genes along the pathway (receptor, ligand and IEGs) can collectively result in defective craniofacial development, including craniofacial clefting. Taken together, these findings provide evidence for a role of the rs28999109 PDGF-C promoter SNP variant in the etiology of CL/P, and highlight the potential importance of regulatory regions in complex traits.6, 47 In addition, the associations seen between other PDGF-C SNPs and CL/P provide further evidence of a role for PDGF-C in some forms of human CL/P.
Acknowledgments
The authors acknowledge and express their sincere appreciation to all individuals who participated in these studies. The staff and collaborators at each study site were critical for successful completion of these studies: Dr. You-e Liu and Dr. Dan-ning Hu (China), Dr. Ajit Ray (India), Dr. Tuncbilek (Turkey), Carla Brandon, Kathy Bardi, Judith Resick, Dr. Katherine Neiswanger, Dr. Joseph Losee (USA—Pittsburgh), Dr. Consuelo Valencia-Ramirez, Dr. Mauricio Camargo, Dr. Mauricio Arcos-Burgos, Dora Rivera (Colombia), Sybill Naidoo, Dr. Rick Martin, Dr. Alex Kane (USA-St. Louis). Operation Smile International, Operation Smile Philippines, the HOPE Foundation, Bill and Kathy Magee, Edith Villanueva, Buena Nepomuceno, Henrietta Gamboa, Salie Onggada, Rachel Lim, and Gloria Melocoton were all critical to sample collection in the Philippines. These studies were supported by National Institutes if Health grants R01-DE09886, R01-DE012472, R37-DE08559, R01-DE016148, R01-DE014667, P50-DE016215; and by the Intramural Program of the National Institute of Dental and Craniofacial Research Z01-DE000711. Some of the genotyping was provided by the Center for Inherited Disease Research (CIDR) which is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University; contract number N01-HG-65403. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health.
Abbreviations
- CL/P
cleft lip with or without cleft palate
- PDGF-C
Platelet-derived growth factor C
- SNP
single nucleotide polymorphism
- Sp1
Specific protein 1
- USF
upstream stimulatory factor
- WT1
Wilms tumor zinc finger protein 1
- EGR-1
early growth response factor 1
- HEB
human B-HLH factor
- ETF
epidermal growth factor receptor (EGFR)-specific transcription factor
References
- 1.Jugessur A, Murray JC. Orofacial clefting: recent insights into a complex trait. Curr Opin Genet Dev. 2005;15:270–278. doi: 10.1016/j.gde.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mossey P. Epidemiology underpinning research in the aetiology of orofacial clefts. Orthod Craniofac Res. 2007;10:114–120. doi: 10.1111/j.1601-6343.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- 3.Juriloff DM, Harris MJ, Brown CJ. Unraveling the complex genetics of cleft lip in the mouse model. Mamm Genome. 2001;12:426–435. doi: 10.1007/s003350010284. [DOI] [PubMed] [Google Scholar]
- 4.Murray JC, Schutte BC. Cleft palate: players, pathways, and pursuits. J Clin Invest. 2004;113:1676–1678. doi: 10.1172/JCI22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieira AR, Avila JR, Daack-Hirsch S, Dragan E, Felix TM, Rahimov F, Harrington J, Schultz RR, Watanabe Y, Johnson M, Fang J, O’Brien SE, Orioli IM, Castilla EE, Fitzpatrick DR, Jiang R, Marazita ML, Murray JC. Medical sequencing of candidate genes for nonsyndromic cleft lip and palate. PLoS Genet. 2005;1:e64. doi: 10.1371/journal.pgen.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahimov F, Marazita ML, Visel A, Cooper ME, Hitchler MJ, Rubini M, Domann FE, Govil M, Christensen K, Bille C, Melbye M, Jugessur A, Lie RT, Wilcox AJ, Fitzpatrick DR, Green E, Mossey PA, Little J, Steegers-Theunissen RP, Pennacchio LA, Schutte BC, Murray JC NISC Comparative Sequencing Program. Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nat Genet. 2008 Oct 5; doi: 10.1038/ng.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewer C, Holloway S, Zawalnyski P, Schinzel A, FitzPatrick DA. Chromosomal deletion map of human malformations. Am J Hum Genet. 1998;63:1153–1159. doi: 10.1086/302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin AE, Garver KL, Diggans G, et al. Interstitial and terminal deletions of the long arm of chromosome 4: further delineation of phenotypes. Am J Med Genet. 1988;31:533–548. doi: 10.1002/ajmg.1320310308. [DOI] [PubMed] [Google Scholar]
- 9.Beiraghi S, Foroud T, Diouhy S, et al. Possible localization of a major gene for cleft lip and palate to 4q. Clin Genet. 1994;46:255–256. doi: 10.1111/j.1399-0004.1994.tb04236.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell LE, Healey SC, Chenevix-Trench G. Evidence for an association between nonsyndromic cleft lip with or without cleft palate and a gene located on the long arm of chromosome 4. Am J Hum Genet. 1995;57:1130–1136. [PMC free article] [PubMed] [Google Scholar]
- 11.Paredes M, Carreno H, Sola JA, Segu J, Palomino H, Blanco R. Association between nonsyndromic cleft lip/palate with microsatellite markers located in 4q. Rev Med Chil. 1999;127:1431–1438. [PubMed] [Google Scholar]
- 12.Marazita ML, Field LL, Cooper, et al. Genome-scan for loci involved in cleft lip with or without cleft palate in Chinese multiplex families. Am J Hum Genet. 2002;71:349–364. doi: 10.1086/341944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding H, Wu X, Bostrom H, et al. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet. 2004;36:1111–1116. doi: 10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- 14.Avila JR, Jezewski PA, Vieira AR, Orioli IM, Castilla EE, Christensen K, Daack-Hirsch S, Romitti PA, Murray JC. PVRL1 variants contribute to non-syndromic cleft lip and palate in multiple populations. Am J Med Genet A. 2006 Dec 1;140(23):2562–70. doi: 10.1002/ajmg.a.31367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L, Caprau D, Christensen K, Suzuki Y, Machida J, Natsume N, Yoshiura K, Vieira AR, Orioli IM, Castilla E, Moreno L, Arcos-Burgos M, Lidral AC, Field LL, Liu Y, Ray A, Goldstein TH, Schultz RD, Shi M, Johnson MK, Kondo S, Schutte B, Marazita ML, Murray JC. Interferon regulatory factor 6 (IRF6) gene variants confer risk for isolated cleft lip and palate. New England Journal of Medicine. 2004;351(8):769–780. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]
- 16.Jezewski PA, Vieira AR, Nishimura C, Ludwig B, Johnson M, O’Brien SE, Daack-Hirsch S, Schultz RE, Weber A, Nepomucena B, Romitti PA, Christensen K, Orioli IM, Castilla EE, Machida J, Natsume N, Murray JC. Complete sequencing shows a role for MSX1 in non-syndromic cleft lip and palate. J Med Genet. 2003;40:399–407. doi: 10.1136/jmg.40.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marazita ML, Murray JC, Lidral AC, et al. Meta-analysis of 13 genome scans reveals multiple cleft lip/palate genes with novel loci on 9q21 and 2q32–35. Am J Hum Genet. 2004;75:161–173. doi: 10.1086/422475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neiswanger K, Deleyiannis FW, Avila JR, et al. Candidate genes for oral-facial clefts in Guatemalan families. Ann Plast Surg. 2006;56:518–521. doi: 10.1097/01.sap.0000210261.65455.9d. [DOI] [PubMed] [Google Scholar]
- 19.Letra A, Menezes R, Granjeiro JM, et al. Defining subphenotypes for oral clefts based on dental development. J Dent Res. 2007;86:986–991. doi: 10.1177/154405910708601013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabe N. AliBaba2: context specific identification of transcription factor binding sites. In Silico Biol. 2002;2:S1–S15. [PubMed] [Google Scholar]
- 21.Choi SJ, Oba T, Callander NS, Jelinek DF, Roodman GD. AML-1A and AML-1B regulation of MIP-1alpha expression in multiple myeloma. Blood. 2003;101:3778–3783. doi: 10.1182/blood-2002-08-2641. [DOI] [PubMed] [Google Scholar]
- 22.Choi SJ, Song IS, Ryu OH, et al. A 4 bp deletion mutation in DLX3 enhances osteoblastic differentiation and bone formation in vitro. Bone. 2008;42:162–171. doi: 10.1016/j.bone.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype--phenotype associations. Eur J Hum Genet. 2001;9:301–306. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- 25.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- 26.Khachigian LM, Williams AJ, Collins T. Interplay of Sp1 and Egr-1 in the proximal platelet-derived growth factor A-chain promoter in cultured vascular endothelial cells. J Biol Chem. 1995;270:27679–27686. doi: 10.1074/jbc.270.46.27679. [DOI] [PubMed] [Google Scholar]
- 27.Midgley VC, Khachigian LM. Fibroblast growth factor-2 induction of platelet-derived growth factor-C chain transcription in vascular smooth muscle cells is ERK-dependent but not JNK-dependent and mediated by Egr-1. Biol Chem. 2004;279:40289–40295. doi: 10.1074/jbc.M406063200. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Ding H. Generation of conditional knockout alleles for PDGF-C. Genesis. 2007;45:653–657. doi: 10.1002/dvg.20339. [DOI] [PubMed] [Google Scholar]
- 29.Han J, Xiao Y, Lin J, Li Y. PDGF-C controls proliferation and is down-regulated by retinoic acid in mouse embryonic palatal mesenchymal cells. Birth Defects Res. 2006;77:438–444. doi: 10.1002/bdrb.20094. [DOI] [PubMed] [Google Scholar]
- 30.Han J, Li L, Zhang Z, Xiao Y, Lin J, Zheng L, Li Y. Platelet-derived growth factor C plays a role in the branchial arch malformations induced by retinoic acid. Birth Defects Res A Clin Mol Teratol. 2007;79:221–230. doi: 10.1002/bdra.20329. [DOI] [PubMed] [Google Scholar]
- 31.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003 Oct;130(20):4769–84. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 32.Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J. 2005;272:5723–5741. doi: 10.1111/j.1742-4658.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- 33.Schmahl J, Raymond CS, Soriano P. PDGF signaling specificity is mediated through multiple immediate early genes. Nat Genet. 2007 Jan;39(1):52–60. doi: 10.1038/ng1922. [DOI] [PubMed] [Google Scholar]
- 34.Chen WV, Delrow J, Corrin PD, Frazier JP, Soriano P. Identification and validation of PDGF transcriptional targets by microarray-coupled gene-trap mutagenesis. Nat Genet. 2004 Mar;36(3):304–12. doi: 10.1038/ng1306. [DOI] [PubMed] [Google Scholar]
- 35.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997 Jul;124(14):2691–700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Pontén A, Aase K, Karlsson L, Abramsson A, Uutela M, Bäckström G, Hellström M, Boström H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000 May;2(5):302–9. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Korzh V, Balasubramaniyan NV, Ekker M, Ge R. Platelet-derived growth factor A (pdgf-a) expression during zebrafish embryonic development. Dev Genes Evol. 2002 Jul;212(6):298–301. doi: 10.1007/s00427-002-0234-3. [DOI] [PubMed] [Google Scholar]
- 38.Eberhart JK, He X, Swartz ME, Yan YL, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet. 2008 Mar;40(3):290–8. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maul RS, Zhang H, Reid JD, 4th, Pedigo NG, Kaetzel DM. Identification of a cell type-specific enhancer in the distal 5′-region of the platelet-derived growth factor A-chain gene. J Biol Chem. 1998;273:33239–33246. doi: 10.1074/jbc.273.50.33239. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Guerrero E, Midgley VC, Khachigian LM. Angiotensin II induction of PDGF-C expression is mediated by AT1 receptor-dependent Egr-1 transactivation. Nucleic Acids Res. 2008;36:1941–1951. doi: 10.1093/nar/gkm923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharnhorst V, Menke AL, Attema J, et al. EGR-1 enhances tumor growth and modulates the effect of the Wilms’ tumor 1 gene products on tumorigenicity. Oncogene. 2000;19:791–800. doi: 10.1038/sj.onc.1203390. [DOI] [PubMed] [Google Scholar]
- 42.Han J, Li L, Zhang Z, Xiao Y, Lin J, Li Y. PDGF-C participates in branchial arch morphogenesis and is down-regulated by retinoic acid. Toxicol Lett. 2006 Oct;25;166(3):248–54. doi: 10.1016/j.toxlet.2006.07.308. [DOI] [PubMed] [Google Scholar]
- 43.Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K. Regulation of fibrogenic/fibrolytic genes by platelet-derived growth factor C, a novel growth factor, in human dermal fibroblasts. J Cell Physiol. 2005 Feb;202(2):510–7. doi: 10.1002/jcp.20154. [DOI] [PubMed] [Google Scholar]
- 44.Reigstad LJ, Martinez A, Varhaug JE, Lillehaug JR. Nuclear localisation of endogenous SUMO-1-modified PDGF-C in human thyroid tissue and cell lines. Exp Cell Re. 2006;312:782–795. doi: 10.1016/j.yexcr.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 45.Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL. SUMO1 haploinsufficiency leads to cleft lip and palate. Science. 2006 Sep 22;313(5794):1751. doi: 10.1126/science.1128406. [DOI] [PubMed] [Google Scholar]
- 46.Pauws E, Stanier P. FGF signaling and SUMO modification: new players in the aetiology of cleft lip and/or palate. Trends Genet. 2007;23:631–640. doi: 10.1016/j.tig.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Tapia-Páez I, Tammimies K, Massinen S, Roy AL, Kere J. The complex of TFII-I, PARP1, and SFPQ proteins regulates the DYX1C1 gene implicated in neuronal migration and dyslexia. FASEB J. 2008 Aug;22(8):3001–9. doi: 10.1096/fj.07-104455. [DOI] [PMC free article] [PubMed] [Google Scholar]