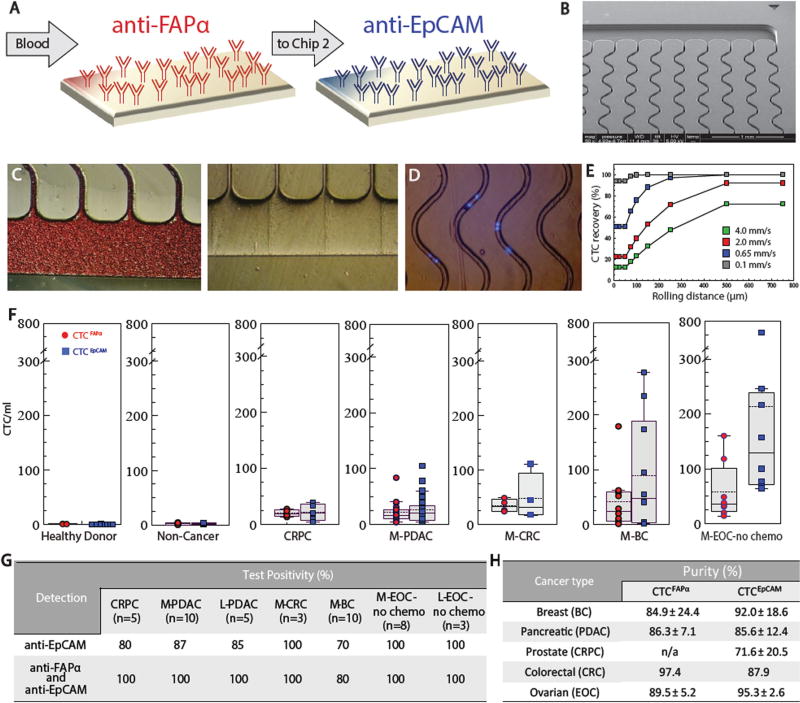
Fig. 1.
Sinusoidal microfluidic device used in the study and summary of clinical results. a Schematic of the dual selection strategy using mAbs directed against FAPα and EpCAM cell-surface antigens. b SEM of the CTC selection microfluidic device. c Optical micrographs of the CTC selection microchip filled with whole blood, and the chip after rinsing with buffer. d An image (5×) of DAPI-stained Hs578T cells isolated within the channels of the microfluidic device. e Simulation of CTC recovery from blood at different translational velocities as a function of cell rolling distance along the mAb decorated surface. f Box plots for CTCs isolated from the blood of healthy donors, patients with non-cancerous disease, CRPC, M- PDAC, M-CRC, M-BC, and M-EOC. CTC counts were normalized to 1ml of blood. g Test positivity in cancer patients’ blood using the single EpCAM approach and the dual selection strategy (test positivity based on the CTCFAPα and/or CTCEpCAM counts exceeding a level that was 3× SD for counts from non-cancer patients)