Abstract
Background
Suicide is a public health concern in the civilian and veteran populations. Stressful life events are precipitating factors for suicide. The neurochemical underpinnings of the association between stress/trauma and suicide risk are unclear, especially in regards to sex differences. We hypothesized that gamma-amino butyric acid (GABA), the major inhibitory neurotransmitter may be a neurochemical candidate that is critical in the association between stress and suicide risk in veterans.
Methods
Proton magnetic resonance spectroscopy (1H MRS) at 3.0 Tesla was used to measure in vivo neurochemistry in the anterior cingulate cortex (ACC; predominantly the dorsal ACC) of 81 veterans (16 females), including 57 (11 females) who endorsed past suicidal ideation (SI) and/or suicide attempt (SA) and 24 (5 females) with no history of SI and/or SA. Suicidal behavior (SB) was defined as the presence of SI and/or SA.
Results
We observed no significant differences in GABA/ Creatine+phosphocreatine (Cr+PCr) between veterans with SB (SB+) and without SB (SB−). However, the female SB+ group showed significantly reduced GABA/Cr+PCr vs. the female SB− group. We observed a trend-level significant negative correlation between GABA/Cr+PCr and the defensive avoidance (DA) subscale on the Trauma Symptom Inventory (TSI) in the SB+ group. In contrast, the SB− group exhibited a positive relationship between the two variables. Furthermore, we found significant negative correlations between GABA/Cr+PCr and Hamilton Rating Scale for Depression (HAM-D) scores as well as between GABA/Cr+PCr and several subscales of the TSI in female veterans.
Conclusions
This study suggests that reduced GABA/Cr+ PCr ratio in the ACC, which may be related to altered inhibitory capacity, may underlie suicide risk in female veterans. Further, the negative association between GABA/Cr+PCr and stress symptomatology and depression scores suggests that MRS studies may shed light on intermediate phenotypes of SB.
Keywords: Stress, Suicide, Veterans, GABA, Magnetic Resonance Spectroscopy
Introduction
Suicide is a significant public health concern worldwide with more people dying by suicide than by homicides and wars combined 1. In 2014, the age-adjusted suicide rate was 13.0 per 100,000 population in the United States 2. While there is considerable debate regarding whether veterans are at an increased risk for suicide compared to the civilian population 3, there is no doubt that death by suicide is also a significant problem in male and female veteran populations. In 2014, an estimated 20 veterans died by suicide each day 4, suggesting a need for novel suicide prevention strategies in the veteran population. Suicide is a multifactorial phenomenon with several risk factors including mental illnesses and stressful/traumatic life events 5, 6. Veterans are exposed to stressful events including combat-related trauma, which may contribute to the risk for suicide 7. Although the biological mechanisms involved in the association between stress and suicide are not clear, converging lines of evidence implicate stress-related changes in brain morphology and neurochemistry in the etiology of suicide 8.
Important sex differences in suicidal behavior (SB) have been reported. For example, females are three times more likely to attempt suicide, but men are four times more likely to die by suicide than women, due in part to the more lethal methods used 9, 10. Further, childhood trauma showed stronger associations with SB in female veterans as compared to males 11. In agreement, women have a higher incidence of stress-related disorders such as post-traumatic stress disorder (PTSD) 12, suggesting that females may be more biologically vulnerable to the effects of stress 13. Given new inclusionary policies, women are increasingly occupying military ranks and serving in combat roles 14. Thus, it is of paramount importance to identify the neurobiological factors underlying the association between combat stress and suicide in order to develop additional suicide prevention strategies.
The gamma-amino butyric acid (GABA)ergic system may underlie the association between stress and SB given that prior evidence has shown links between GABA and stress responses as well as suicide 15–18. GABA-related alterations in suicide have been reported at the neurochemical, cellular, and genetic levels 15. For instance, quantitative polymerase chain reaction (PCR) studies showed that GABA-A receptor organization was altered in the frontal cortex, hippocampus, and amygdala in depressed individuals who died of suicide as compared to those who died due to other causes 19, 20. Moreover, microarray-based investigations have revealed alterations in several GABAergic genes on a global level in the brain of depressed suicides 21, 22. Furthermore, rodent and human studies have demonstrated stress-induced alterations in GABA transmission in the frontal cortex. For instance, chronic stress alters GABA-A receptor as assessed by radioligand binding 23, decreases GABA synthesizing enzymes and GABA bioavailability, and impairs function of specific types of GABA interneurons in the cortex in rodent models 24. In humans, a positron emission tomography (PET) study showed reduced GABA-A receptor binding in veterans with deployment-related PTSD 25. Furthermore, lower serum GABA after trauma exposure predict development of PTSD 26. Finally, a recent study reported that the GABRA6 gene (encodes the GABA-A receptor alpha-6 subunit) variant plays an important role in mediating the effects of recent stress in the development of suicidal risk-related phenotypes 27. Specifically, GABRA6 T carriers showed an increased risk of specific elements of suicide risk after exposure to stressful life events 27. Importantly, in the absence of stress, GABRA6 T carriers did not exhibit an increased risk for suicide-related phenotypes 27. Collectively, these studies highlight that GABAergic transmission is dysregulated after stress as well as in suicide; and evidence suggests a complex interaction between the GABAergic system and stress associated with phenotypes related to SB 27.
There exist important sex differences with regard to stress-induced changes in GABAergic transmission 28. For example, female rats show an increase in low-affinity GABA binding sites after swim stress but males do not 29, 30. In humans, either a decrease 31 or no change 32 in frontal GABA has been reported in response to acute psychological stress using proton magnetic resonance spectroscopy (1H-MRS). Interestingly, the study that reported no changes in GABA concentration after stress examined only male subjects 32 while the study reporting a decrease included both male and female subjects 31. Sex differences in stress-induced alterations in the GABAergic system may be partly explained by differential levels of neurosteroids, which are under the control of ovarian hormones and play an important role in the GABAergic regulation of the hypothalamic-pituitary-adrenal (HPA) axis stress response 33. Overall, these studies imply that there may be sex-specific differences in stress effects on GABA, which may influence the vulnerability of development of stress-related neurobiological disorders including SB.
1H-MRS is an increasingly used non-invasive technique to measure in vivo neurochemistry 34. Recent developments in the technique have made it possible to individually quantify glutamate, glutamine, and GABA, which is unreliable without specialized methods 35–37. We examined GABA/Cr+PCr differences between SB+ and SB− groups in the ACC since this region has been implicated in imaging studies of suicide. For example, reduced ACC gray matter density measured by voxel-based morphometry was observed in MDD patients at high risk for suicide when compared to non high-risk MDD patients 38. In addition, a meta-analysis showed increased ACC activation during emotional tasks and reduced ACC activation during cognitive tasks to be associated with SB 39. In a study of combat-exposed veterans, those with SI showed more engagement of the ACC during error processing as compared to veterans without SI 40.
The purpose of the current study was to characterize the association between SB, stress and GABAergic transmission in the ACC using the 1H-MRS technique in male and female veterans. On the basis of evidence supporting a role for GABA in stress and suicide 24, 26,31, we specifically hypothesized that veterans with SB (SB+) would exhibit lower GABA/Cr+PCr than veterans without SB (SB−). We further explored whether between group difference in GABA/Cr+PCr is related to stress response and sex.
Methods
Participants
Eighty-one veterans (16 females) were enrolled in the study. Participants were recruited from a local VA hospital as well as from the community via flyers. The Institutional Review Boards at the University of Utah and the George E. Wahlen Department of Veterans Affairs (VA) Medical Center approved this study. All subjects provided written informed consent as per the IRB and Declaration of Helsinki. Participants were compensated financially for their time. Combat and non-combat exposed veterans between the ages of 18 and 55, male or female, and of any race or ethnicity were included. Veterans with a history or current diagnosis of depression, PTSD, and substance use disorder were included. Further, we included veterans who were stable on current psychotropic medication regimen for more than 3 months. While it acknowledged that medications may have potential confounding effects the research team felt it would be unethical to ask participants to discontinue medication for the study; therefore, we included participants who were on antidepressants, anxiolytics, mood stabilizers and antipsychotic medications. Exclusion criteria included major sensorimotor handicaps, estimated full scale IQ<80, history of autism, claustrophobia, electroconvulsive therapy, significant medical or neurological illness that may affect cognitive function, with the exception of traumatic brain injury (TBI), and any MRI contraindications. Pregnant or lactating females were excluded. Further, the menstrual cycle was not assessed and laboratory assessments for hormone levels were not completed.
Procedures
Participants completed the Structured Clinical Interview for DSM-IV-TR (SCID-IV-TR), a clinician administered, semi-structured interview to determine general health functioning (GAF) as well as current and past mental health diagnoses 41. The Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II) 42 was administered to all participants to assess estimated IQ.
Participants also completed a clinical battery including the Columbia Suicide Severity Rating Scale (C-SSRS) 43, Hamilton Rating Scale for Anxiety and Depression (HAM-A, HAM-D) and the Trauma Symptom Inventory (TSI) 44, 45. Finally, participants completed a 1H-MRS scan at the visit.
The C-SSRS assesses lifetime presence of SI, plans, intensity of ideation and SA. SA includes an actual attempt, an interrupted attempt, or an aborted/self-interrupted attempt. Constructs on the C-SSRS have been found to be acceptable internal consistency as well as convergent, divergent, and predictive validity and predict SA in a 24-week follow-up period 43.
The TSI scale 46 is a widely used, 100 item self-report measure developed to assess post-trauma symptoms 47. The TSI is used in the evaluation of acute and chronic posttraumatic symptomatology, including the sequelae of rape, spouse abuse, physical assault, combat experiences, major accidents, and natural disasters, as well as the lasting sequelae of childhood abuse and early traumatic events. The 10 TSI scales are the following: Anxious Arousal (AA), Anger Irritability (AI), Depression (D), Dissociation (Dis), Defensive Avoidance (DA), Intrusive Experiences (IE), Tension Reduction Behavior (TRB), Impaired Self-reference (ISR), Sexual Concerns (SC), Dysfunctional Sexual Behavior (DSB). The TSI scale demonstrates good internal consistency and convergent validity in civilian as well as veteran populations 48–50.
The HAM-A and HAM-D are widely used rating scales to measure the severity of anxiety and depressive symptoms respectively 44. It is based on the clinician’s interview with the patient and probes symptoms such as depressed mood, guilty feelings, suicide, sleep disturbances, anxiety, and weight loss. Research has demonstrated a validity coefficient of .85 51. The HAM-A is a rating scale developed to quantify the severity of anxiety symptomatology, and it is often used in psychotropic drug evaluations 45.
Magnetic Resonance Spectroscopic Imaging: Acquisition and Analysis
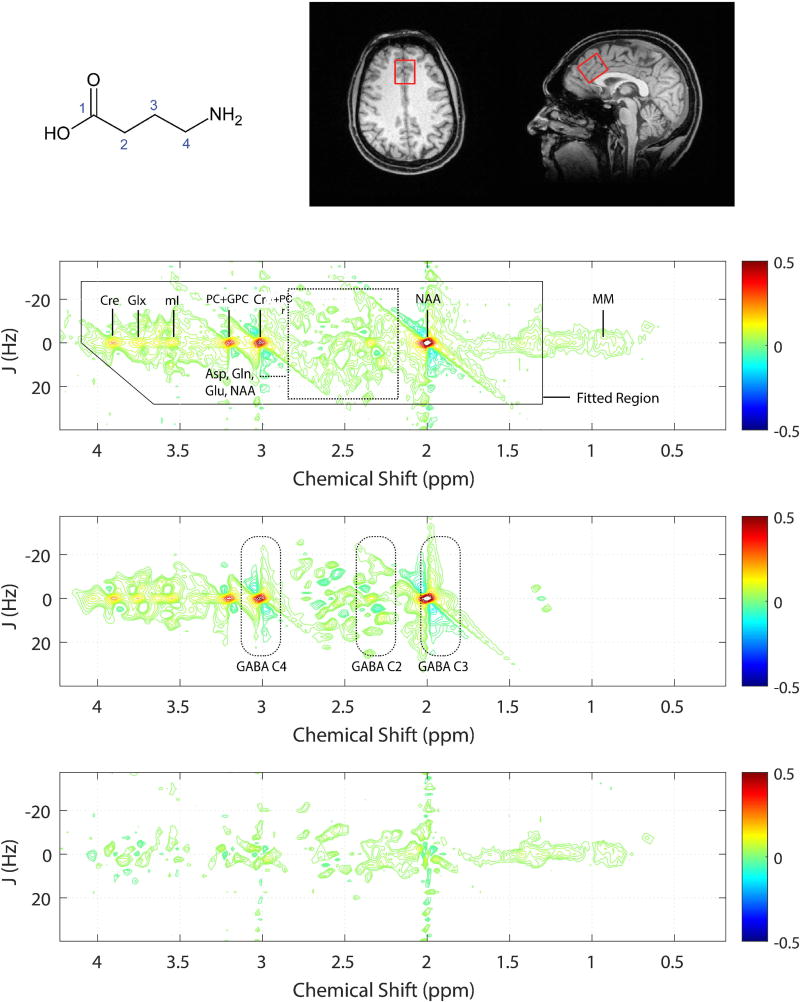
1H-MRS measurements were performed using a 3.0 Tesla Siemens (Erlangen, Germany) Verio™ whole-body MRI scanner. Three-dimensional, high-resolution, magnetization-prepared, rapid gradient echo (MP-RAGE) MR images (TR/TE/TI = 2000/3.53/1100 ms; FOV = 256×256×224 mm; 1 mm isotropic resolution) were obtained to facilitate the positioning of an obliqued MRS voxel (25 × 25 ×30 mm3) within predominantly gray matter of the ACC. The 1H-MRS voxel was placed midline to primarily cover the dorsal anterior cingulate cortex on midsagittal T1-weighted images, with the anterior ventral edge of the voxel aligned with the centroid of the genu of the corpus callosum (Fig. 1). The MRS voxel was obliqued along the sagittal plane with its smallest dimension spanning the anterior–posterior axis and the largest dimension in the superior-inferior orientation.
Figure 1.
Top left: GABA structure, Top right: Axial and mid-sagittal slices extracted from a tissue-segmented 3D MP-RAGE MRI dataset recorded from a single subject. Red rectangle depicts the positioning of the MRS voxel in the ACC. Fitted (top), raw (middle) and residuals (bottom) 2D-J 1H-MRS spectra analyzed using Prior Knowledge Fitting (ProFit). Dashed boxes indicate the 2D spectral regions where the GABA protons resonate. The color bars to the right show contouring amplitudes and signal phase. The raw data displayed shows tentative signal assignments for the dominant metabolite resonances as well as macromolecules (MM).
Within-voxel B0 shimming was achieved using a manufacturer-supplied phase map procedure in combination with interactive manual shimming until a full-width at half-maximum (FWHM) of ≤ 11 Hz was observed for the real component of the ACC unsuppressed water signal. A PRESS sequence was used to acquire two-dimensional (2D) J-resolved 1H MRS spectra measurements, modified to enable TE stepping: TR/TE range = 2400 / 31–229 ms; signal averages per TE = 4; deltaTE = 2 ms; 3-pulse WET water suppression. The spectral data were obtained using a maximum-echo sampling scheme whereby the analogue-to-digital converter (ADC) on-time was fixed for all 100 TE steps 52. Outer-volume suppression (OVS) was achieved using six saturation bands positioned at least 1.5-cm away from the MRS voxel faces and band saturation was achieved using hyperbolic secant adiabatic full passage RF pulses. A three-pulse water elimination through T1-effects (WET 53) scheme was interleaved with the OVS module for global water suppression. An additional water unsuppressed 2D J-resolved 1H MRS dataset was recorded from each voxel with 2 signal averages recorded for each TE step.
Tissue segmentation
To control for within-voxel tissue variability, skull-stripping and brain tissue-type segmentation was applied to all MP-RAGE images using the Brain Extraction Tools 54 and FAST 55 tools provided with the freely-available FMRIB software library 56. MATLAB (TheMathWorks, Natick, MA) was used to extract the 3D volume corresponding to the positioned MRS voxel and calculate within-voxel gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue content for each subject. The within-voxel GM % was calculated as the ratio to the total brain matter, i.e., 100 X GM/(GM+WM).
MRS data processing
All 2D J-resolved 1H MRS data were quantified using the prior knowledge fitting (ProFit) algorithm without additional line broadening applied prior to spectral fitting. Before the 2D fast Fourier transformation (FFT), the raw 2D matrix was zero-filled to 200 points along the indirectly detected J dimension. The ProFit algorithm fits basis spectra from a total of nineteen metabolites to the raw 2D spectral surface without considering the effects of spatial localization 52, 57. The basis set comprised of N-acetylaspartate (NAA), glycerophosphocholine (GPC), phosphorylcholine (PC), alanine (Ala), aspartate (Asp), glucose (Glc), glycine (Gly), lactate (lac), N-acetylaspartylglutamate (NAAG), ascorbic acid (Asc), phosphoethanolamine (PE), taurine (Tau), scyllo-inositol (sI), total creatine (Cr+PCr), glutamate, glutamine, GABA, myoinositol, and glutathione (GSH). Using identical 2D 1H MRS methodology in 10 healthy volunteers, we previously have reported a within-subject coefficient of variation (CV) of 15 % and a between-subject CV of 24% for ACC GABA normalized to water58. All metabolites were expressed as metabolite/water ratios and corrected for the within-voxel CSF fraction using segmented MRI data as previously described 58,35. All fitted metabolite areas were also normalized to total Cre (Cr+PCr). Metabolite/water and metabolite/Cr+PCr ratios thus are expressed as institutional units (I.u) and presented as the mean ± standard error mean (SEM).
Statistical analysis
Group differences in demographic, clinical and MRS measures between veterans with and without SB were evaluated using Student’s t-test. One-way ANCOVA was used to adjust for age and sex when analyzing differences in demographic and clinical variables. Pearson’s tests were used to assess relationships between clinical variables and neurochemical levels. Further, partial correlations were run with age as covariate. We conducted the correlation analysis in the combined sample rather than segregating participants by SB since symptoms of depression, anxiety, and stress may also exist in the SB− group. This is in line with the Research Domain Criteria (RDoC) approach 59 whereby behavioral constructs are evaluated along a continuum and not by diagnostic categories, which has increasingly been used in several studies 60, 61. Non-parametric tests (Mann-Whitney U test and Spearman’s correlation) were used if the measures failed test for normality, which was tested using the Shapiro-Wilk’s test. The correlation analyses were exploratory in nature given the small sample size for female veterans and hence we did not correct for multiple comparisons. Thus, our hypothesis testing should be considered preliminary and not definitive. We used G*Power 3.1 to compute posthoc achieved power given an alpha=0.05 and our sample size. All other analyses were performed in SPSS 20 (IBM, Chicago, IL). p values less than 0.05 were considered significant.
Results
Clinical and demographic variables
Clinical and demographic characteristics for each group are shown in Table 1. There were no significant differences between SB+ and SB− groups with regard to age, education and IQ. Further, when adjusted for sex and age, IQ and education were not significantly different between the two groups. When analyzed separately, the female SB+ group did not differ significantly from the female SB− group with regard to age, education and IQ (p>0.05). As expected, the SB+ group had significantly higher scores of depression and anxiety on the HAM-D and HAM-A respectively, as compared to the SB− group. The scores on each of the TSI subscales were also significantly different between the two groups (SB+>SB−). When sex and age were included as covariates, the difference in HAM-A, HAM-D, and TSI subscales between the two groups continued to remain significant (p<0.05).
Table 1.
Demographic and clinical variables represented as mean ± S.D. (WASI=Wechsler Abbreviated Scale for Intelligence, HAM-D=Hamilton Rating Scale for Depression, HAM-A= Hamilton Rating Scale for Anxiety, TSI=Trauma Symptom Inventory, AA= Anxious Arousal, D=Depression, AI=Anger Irritability, TRB= Tension Reduction Behavior, IE= Intrusive Experience, DA=Defensive Avoidance, Diss= Dissociative Behavior, ISR= Impaired Self-Reference)
| SB+ | SB− | p-value | |
|---|---|---|---|
| Sex | 46 males, 11 females | 19 males, 5 females | |
| Age | 37.2 ± 9.1 | 36.2 ± 9.7 | 0.62 |
| Education | 15 ± 2.2 | 14.7 ± 1.7 | 0.57 |
| WASI-IQ | 110.8 ± 9.6 | 114.3 ± 11.1 | 0.16 |
| HAM-D | 9.8 ± 7.5 | 1.9 ± 2.5 | <0.001** |
| HAM-A | 10.1 ± 8.3 | 3.0 ± 4.1 | <0.001** |
| TSI-AA | 58.8 ± 11.7 | 48.1 ± 8.6 | <0.001** |
| TSI-D | 60.4 ± 11.5 | 46 ± 7.1 | <0.001** |
| TSI-AI | 56.7 ± 11.7 | 44.4 ± 6 | <0.001** |
| TSI-TRB | 58.3 ± 14 | 47.1 ± 4.3 | <0.001** |
| TSI-IE | 62.3 ± 12.5 | 50.6 ± 5.9 | <0.001** |
| TSI-DA | 59.9 ± 11.1 | 47.6 ± 6.2 | <0.001** |
| TSI-Diss | 59 ± 11.9 | 49.7 ± 7 | <0.001** |
| TSI-ISR | 58.7 ± 10.5 | 45.4 ± 5.5 | <0.001** |
Tissue segmentation
Figure 1 displays tissue-segmented axial and sagittal images extracted from a 3D MP-RAGE dataset recorded from a single HC subject. Table 2 displays the within-voxel GM and WM content for both subject cohorts. We did not find significant differences between the two groups. Further, the CSF content within the ACC did not differ between the two groups (p=0.42). Within female veterans, the GM, WM, and CSF content did not differ significantly between the SB+ and SB− groups.
Table 2.
Tissue Fractions (GM and WM) calculated for both groups and brain regions under investigation. Values are expressed as expressed as group mean % fraction ± SD.
| Brain Region | Group | GM | WM | p-value |
|---|---|---|---|---|
|
| ||||
| ACC | SB+ | 69.44 ± 5 | 30.55 ± 5 | 0.52 |
| SB− | 70.22 ± 3 | 29.78 ± 3 | ||
Group differences in metabolite ratios
Cr+PCr/H2O ratio did not exhibit significant differences between groups. When data was analyzed separately for females, we did not observe significant differences in Cr+PCr/H2O ratios between the SB+ and SB− groups. This was also true for when age was added as a covariate. Thus, Cr+PCr was used as a non-biased internal standard (denominator) and GABA/Cr+PCr was used as an outcome measure for this study.
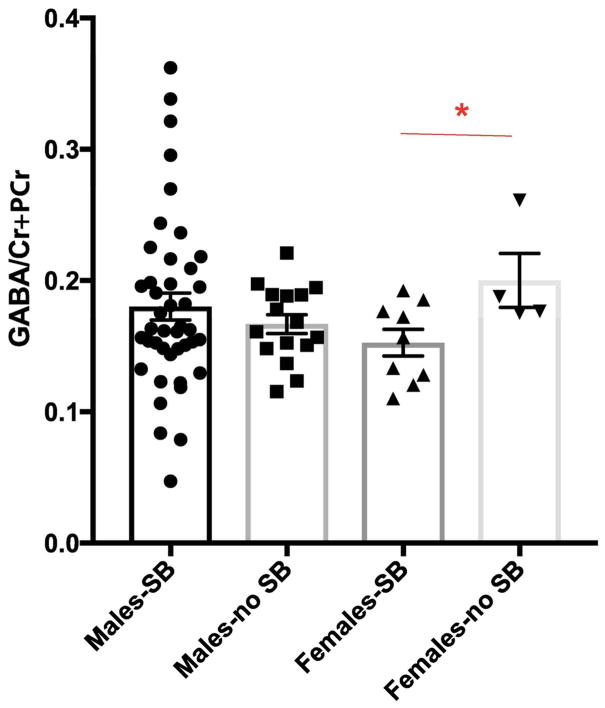
No significant differences were found in the levels of GABA/Cr+PCr (t=0.12, p=0.9, Table 3) between SB+ and SB− groups. Ratios of other key metabolites are also reported (Tables 3, S1). However, when data was analyzed separately for males and females, we observed that female veterans with SB had lower GABA/Cr+PCr as compared to female veterans without SB (t-ratio=−2.35, p=0.039, Fig. 2, Table 3). When age was added as a covariate, the p value approached significance (p=0.06).
Table 3.
Key metabolite concentrations normalized to creatine in the ACC (mean ± standard error)(Institutional units).
| Male-SB+ | Male-SB− | Female-SB+ | Female-SB− | p value (SB+ vs.SB−) | p value (males SB+ vs.males SB−) | p value (females SB+ vs.females SB−) | |
|---|---|---|---|---|---|---|---|
| NAA/Cr+PCr | 1.34 ± 0.02 | 1.36 ± 0.04 | 1.41 ± 0.03 | 1.40 ± 0.07 | 0.79 | 0.76 | 0.92 |
| GABA/Cr+PCr | 0.18 ± 0.01 | 0.17 ± 0.007 | 0.15 ± 0.01 | 0.2 ± 0.02 | 0.9 | 0.44 | 0.04* |
| Gln/Cr+PCr | 0.26 ± 0.01 | 0.27 ± 0.03 | 0.22 ± 0.02 | 0.25 ± 0.006 | 0.35 | 0.49 | 0.37 |
| Glu/Cr+PCr | 1.12 ± 0.02 | 1.12 ± 0.03 | 1.15 ± 0.03 | 1.12 ± 0.12 | 0.99 | 0.86 | 0.73 |
NAA: N-Acetylaspartate, Cr+PCr: Creatine+phosphocreatine, GABA: Gamma amino butyric acid, Gln: Glutamine, Glu: Glutamate,
indicates significance (t-test)
Figure 2.
The GABA/Cr+PCr ratio was significantly lower in female veterans who endorsed SB when compared to those who did not. * indicates p<0.05. Data is represented as mean ± S.E.M.
Correlation between GABA/Cr+PCr and clinical characteristics
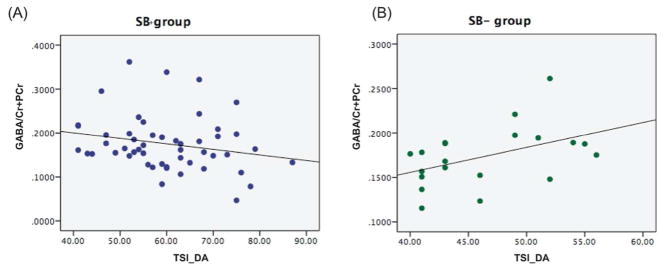
In the SB+ group, we observed a trend towards a negative correlation between GABA/ Cr+PCr and TSI-DA subscale (Spearman’s rho=−0.27, p=0.06, Fig. 3A). Interestingly, the relationship between GABA/Cr+PCr and the TSI-DA subscale was opposite in the SB− group (Spearman’s rho=0.44, p=0.05, Fig. 3B). When age was included as a covariate, the relationship between GABA/Cr+PCr and the TSI-DA subscale approached significance in the SB+ (Spearman’s rho=−0.25, p=0.08) group; however, the significant relationship was lost in the SB− group (Spearman’s rho=0.35, p=0.1).
Figure 3.
Correlation of GABA/Cr+PCr and Defensive Avoidance (DA) subscale on the Trauma Symptom Inventory (TSI) measure in (A) SB+ group (B) SB− group.
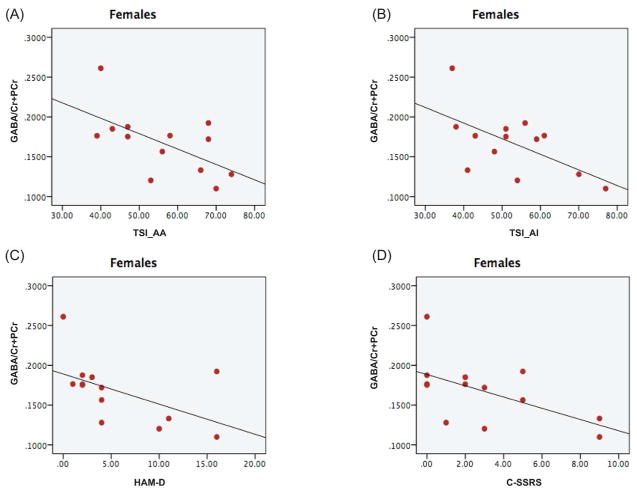
Since we observed significant group differences in GABA/Cr+PCr ratio in female veterans, we performed an exploratory analysis to investigate any potential relationship between GABA and clinical variables in female veterans. Significant negative relationships were observed between GABA/Cr+PCr and TSI-AA (Pearson’s correlation coefficient=−0.6, p=0.03, Fig. 4A), TSI-AI (Pearson’s correlation coefficient=−0.596, p=0.03, Fig. 4B), HAM-D (Pearson’s correlation coefficient=−0.576, p=0.04, Fig. 4C), and C-SSRS scores (Spearman’s rho=−0.53, p=0.05, Fig. 4D). With age as a covariate, the relationship between GABA/Cr+PCr and TSI-AA (Pearson’s correlation coefficient=−0.66, p=0.02), TSI-AI (Pearson’s correlation coefficient=−0.79, p=0.002) continued to remain significant while the correlation between GABA/Cr+PCr and HAM-D (Pearson’s correlation coefficient=−0.54, p=0.07), and C-SSRS scores (Pearson’s correlation coefficient=−0.53, p=0.07) showed a trend towards significance. Further, the negative association between GABA/Cr+PCr and HAM-A (Spearman’s rho=−0.48, p=0.09), TSI-DA (Spearman’s rho=−0.52, p=0.07) approached significance in our cohort of female veterans. With age included as a covariate, the association between GABA/Cr+PCr and the TSI-DA subscale (Spearman’s rho=−0.57, p=0.05) was significant while the GABA/ Cr+PCr and HAM-A correlation (Spearman’s rho=−0.5, p=0.09) continued to show a modest trend towards significance.
Figure 4.
Correlation of GABA/Cr+PCr and (A) Anxious Arousal (AA) subscale (B) Anger Irritability Subscale (AI) of the TSI measure (C) HAM-D scores (D) C-SSRS in female veterans.
Power analysis
The final sample size had high power (1-β >0.90) to detect 1 large (d=0.8) and medium effect sizes (d=0.5) and medium power (1-β =0.72) to detect a small effect size (d=0.3).
Discussion
To the best of our knowledge, this is the first 1H-MRS study in the ACC in veterans with SB. Specifically, we observed decreased ACC GABA/Cr+PCr in the female SB+ group (vs. female SB− group). Our results also indicated opposite direction of correlation between GABA/Cr+PCr and the TSI-DA in veterans with and without SB, though the correlation in SB+ only approached significance. In addition, we found significant negative correlations between GABA/Cr+PCr and clinical variables such as HAM-D, TSI-AI, TSI-AA, and C-SSRS scores in female veterans, suggesting that GABA/Cr+PCr in ACC may be sensitive to variation along a continuum of subclinical to pathologic depressive and stress symptoms. Caution is warranted in interpreting the findings given the small sample size of female veterans. Further, the ratio of male to female veterans in the study was significantly skewed towards males. These findings will have to be replicated in a larger cohort of female veterans as well as in a sample of equally distributed male and female veterans to draw firm conclusions.
Nonetheless, these results add to a burgeoning body of literature that has suggested a prominent role for GABA dysregulation in suicide 15. GABA, the major inhibitory neurotransmitter is important in modulating excitation in the brain by controlling the firing rate of intrinsic cortical neurons 62. Studies have reported alterations in GABA receptor subunit genes, as well as down regulation of GABA-A receptor in postmortem brain analyses of suicide decedents 19, 20. Despite this evidence, studies reporting on in vivo brain GABA in individuals at high risk for suicide are scarce. Thus far, only two 1H-MRS studies have investigated in vivo brain GABA in individuals with SB 63, 64 but none involved a veteran population. Moreover, neither of the studies reported significant alterations in GABA/Cr+PCr in those with SB. The brain regions of interest in these studies were the dorsal prefrontal cortex 63 and the hippocampus 64 and neither of the studies analyzed MRS data separately by sex. Thus it may be possible that reduced GABA/Cr+PCr in female veterans with SB observed in the current study is specific to the ACC and the female sex. Importantly, we did not observe alterations in NAA/H2O and Cre/H2O, which are often seen in pathologies involving neuronal loss 65–67. Thus, reduction in GABA may not be secondary to cell loss but instead may involve abnormalities in GABA synthesis and metabolism. Overall this is the first report to suggest that reduced ACC GABA/Cr+PCr ratio may play a role in heightened suicide risk in female veterans.
ACC GABA/Cr+PCr showed a trend-level negative correlation with the TSI-DA subscale in the SB+ group as opposed to a positive relationship between the two variables in the SB− group. The DA subscale measures posttraumatic cognitive and behavioral avoidance. Thus, the above results suggest that stress/trauma shows a trend towards affecting ACC GABA/Cr+PCr differently in veterans with and without SB, which may underlie higher scores of TSI-DA in veterans with SB.
Dysfunction of the GABAergic system has been implicated in depression, which is related to stress-related psychopathology and SB 15. In support, we observed that ACC GABA/Cr+PCr ratios were inversely correlated with C-SSRS, TSI-AA, TSI-AI, and HAM-D scores in females. A negative correlation between C-SSRS scores and GABA is concordant with reduced GABA in female veterans with SB. The TSI-AA scale taps into symptoms of anxiety, including those associated with posttraumatic hyperarousal and the TSI-AA scale measures angry or irritable affect in the context of trauma 48. Structural and functional neuroimaging studies have consistently implicated the ACC in mediating anxiety 68, hyperarousal and emotion dysregulation symptoms in response to stress/trauma 69, 70. Thus, it seems logical to find a relationship between ACC neurochemistry and psychological mechanisms that influence responses to stress/trauma. Moreover, we also observed a negative correlation between GABA/Cr+PCr and HAM-D scores, which is largely consistent with the literature implicating GABAergic dysfunction in major depression. For example, MRS studies in depressed adults and adolescents exhibited GABA deficits in the brain 61, 71. Decreased GABA has also been observed in the plasma 72 and CSF 73 of depressed individuals. Importantly, lower GABA has been associated with treatment resistant depression 74, which is characterized by more severe outcomes such as suicide. Finally, a recent study in depressed patients by Brennan and colleagues showed a significant association between the clinical response to citalopram at day 42 with a greater increase (or lesser decrease) in GABA/Cr+PCr in the ACC from baseline to day 3 and day 7 of treatment 75. Thus, an antidepressant response is associated with early increases in GABA in the ACC, implicating reduced ACC GABA in the etiology of depressive symptoms. Taken together, these results suggest that deficits in ACC GABA associated with increased stress and depressive symptomatology may be a potential neurobiological explanation for the role of stress and trauma in promoting SB, at least in females.
The current study suggests that there may exist sex differences in the role of GABAergic transmission in SB; however, findings need to be replicated in a larger cohort. We found reduced GABA/Cr+PCr in the ACC in the female SB+ group when compared to the female SB− group. However, this finding was not observed for male veterans. Women, especially veterans have been under-represented in suicide research till date. Genetic and neurobiological differences may exist in SB between males and females. For instance, a genomics study investigating genes that change in expression between women with no SI and women with high SI showed that a number of biomarkers for SI change in the opposite direction than observed in men 76. Sex differences in neurobiology underlying suicidality merits more attention and may be a first step in the direction of individualized/personalized medicine.
Several limitations need to be considered in the interpretation of the results. First, the sample size of female veterans was small, therefore the findings must be considered preliminary and the results must be replicated in studies with larger sample sizes and multiple testing procedures. The present study remains subject to the possibility of Type 1 error and larger populations will allow more rigorous statistical testing. Second, GABA neurotransmission is tightly regulated by the menstrual cycle in females 77. The current study did not match female participants for menstrual cycle stage. Thus, it is possible that menstrual cycle stage may have affected the differences in GABA/Cr+PCr between the two groups. Third, veterans in the current study were not asked to stop taking prescription medications for ethical reasons and some participants suffered from substance use. Thus, we cannot rule out the confounding effects of medications and drugs of abuse on the observed differences. Fourth, GABA is an important neurochemical correlate of depression 78, anxiety 79, and PTSD 80, and the current cohort included participants with these disorders. Thus, the presence of past and/or current co-morbidities within the two groups may be potential confounds in the present study. Future studies should address this limitation by including both patient control and healthy control groups and rigorous assessments of illness severity across diagnostic domains. This study design will enable us to determine which neurochemical changes are due to the diathesis for SB and which are related to primary psychiatric disorders. Finally, the coefficient of variance (CV) for metabolite ratios to Cr+PCr in the ACC were higher than previously reported 52, which may reflect the heterogeneity of the population and hence the negative findings for other metabolites should be interpreted with caution.
In summary, this pilot study reports three preliminary findings. First, 1H-MRS GABA/Cr+PCr in the ACC was reduced in female veterans with SB. Second, the direction of association between GABA/Cr+PCr and the TSI-DA was opposite in SB+ and SB− groups. Finally, in females, a number of TSI subscales and HAM-D showed a negative correlation with ACC GABA/Cr+PCr. The current study motivates future investigations into sex differences in the neurobiological underpinnings of SB as well as the association between stress/trauma and SB in a larger cohort of female veterans.
Supplementary Material
Acknowledgments
Funding: This manuscript is based upon work supported by the Department of Veterans Affairs, but does not necessarily represent the views of the Department of Veterans Affairs or the United States Government. This presentation is based upon work supported with resources and the use of facilities at the VISN 19 MIRECC. This project is funded by the Military Suicide Research Consortium (MSRC), an effort supported by the Office of the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-10-2-0178; and through Merit Review 5I01CX000253-02.
Footnotes
Declaration of Conflicting Interests: The authors declare no conflict of interest
References
- 1.Nations U. World Population Prospects: The 2008 Revision, Vol. 1, Comprehensive Tables and United Nations, Highlights. Popul Dev Rev. 2009;36:854–855. [Google Scholar]
- 2.Curtin SCWM, Hedegaard H. NCHS data brief, no 241. Hyattsville, MD: National Center for Health Statistics; 2016. Increase in suicide in the United States, 1999–2014. [Google Scholar]
- 3.McGlade E, Bakian A, Coon H, et al. Male suspected suicide decedents in Utah: A comparison of Veterans and nonveterans. Comprehensive psychiatry. 2016 Jul 18;69:1–10. doi: 10.1016/j.comppsych.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Administration. OoSPiVH. Suicide among Veterans and other Americans, 2001–2014. 2016. [Google Scholar]
- 5.Bryan CJ, Clemans TA, Leeson B, et al. Acute vs. chronic stressors, multiple suicide attempts, and persistent suicide ideation in US soldiers. The Journal of nervous and mental disease. 2014 Dec 17;203:48–53. doi: 10.1097/nmd.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 6.Santa Mina EE, Gallop RM. Childhood sexual and physical abuse and adult self-harm and suicidal behaviour: a literature review. Canadian journal of psychiatry Revue canadienne de psychiatrie. 1998 Nov 07;43:793–800. doi: 10.1177/070674379804300803. [DOI] [PubMed] [Google Scholar]
- 7.Lemaire CM, Graham DP. Factors associated with suicidal ideation in OEF/OIF veterans. Journal of affective disorders. 2010 Nov 09;130:231–238. doi: 10.1016/j.jad.2010.10.021. 2011. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig B, Roy B, Wang Q, et al. The Life Span Model of Suicide and Its Neurobiological Foundation. Frontiers in neuroscience. 2017 Mar 07;11:74. doi: 10.3389/fnins.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maris RW, Berman AL, Silverman MM. Suicide, gender and sexuality. In: Maris RW, Berman AL, Silverman MM, editors. Comprehensive textbook of suicidology. New York: Guilford; 2000. pp. 145–169. [Google Scholar]
- 10.Hoffmire CA, Kemp JE, Bossarte RM. Changes in Suicide Mortality for Veterans and Nonveterans by Gender and History of VHA Service Use, 2000–2010. Psychiatric services (Washington, DC) 2015 May 02;66:959–965. doi: 10.1176/appi.ps.201400031. [DOI] [PubMed] [Google Scholar]
- 11.Benda BB. Gender differences in predictors of suicidal thoughts and attempts among homeless veterans that abuse substances. Suicide & life-threatening behavior. 2005 Apr 22;35:106–116. doi: 10.1521/suli.35.1.106.59262. [DOI] [PubMed] [Google Scholar]
- 12.Oquendo MA, Friend JM, Halberstam B, et al. Association of comorbid posttraumatic stress disorder and major depression with greater risk for suicidal behavior. The American journal of psychiatry. 2003 Mar 04;160:580–582. doi: 10.1176/appi.ajp.160.3.580. [DOI] [PubMed] [Google Scholar]
- 13.Carter-Snell C, Hegadoren K. Stress disorders and gender: implications for theory and research. The Canadian journal of nursing research = Revue canadienne de recherche en sciences infirmieres. 2003 Aug 12;35:34–55. [PubMed] [Google Scholar]
- 14.Conard PL, Sauls DJ. Deployment and PTSD in the female combat veteran: a systematic review. Nursing forum. 2014 Jan 25;49:1–10. doi: 10.1111/nuf.12049. [DOI] [PubMed] [Google Scholar]
- 15.Pabba MSE. Biological Aspects of Suicidal Behavior. 2016. GABA, Depression and Suicide. [Google Scholar]
- 16.Hasler G, Nugent AC, Carlson PJ, et al. Altered cerebral gamma-aminobutyric acid type A-benzodiazepine receptor binding in panic disorder determined by [11C]flumazenil positron emission tomography. Archives of general psychiatry. 2008 Oct 08;65:1166–1175. doi: 10.1001/archpsyc.65.10.1166. [DOI] [PubMed] [Google Scholar]
- 17.Cullinan WE, Wolfe TJ. Chronic stress regulates levels of mRNA transcripts encoding beta subunits of the GABA(A) receptor in the rat stress axis. Brain research. 2001 Jan 03;887:118–124. doi: 10.1016/s0006-8993(00)03000-6. 2000. [DOI] [PubMed] [Google Scholar]
- 18.Gronli J, Fiske E, Murison R, et al. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behavioural brain research. 2007 May 05;181:42–51. doi: 10.1016/j.bbr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004 Feb 13;24:1478–1485. doi: 10.1523/jneurosci.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulter MO, Du L, Zhurov V, et al. Altered Organization of GABA(A) Receptor mRNA Expression in the Depressed Suicide Brain. Frontiers in molecular neuroscience. 2010 Apr 22;3:3. doi: 10.3389/neuro.02.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klempan TA, Sequeira A, Canetti L, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Molecular psychiatry. 2007 Oct 17;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- 22.Sequeira A, Mamdani F, Ernst C, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PloS one. 2009 Aug 12;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drugan RC, Morrow AL, Weizman R, et al. Stress-induced behavioral depression in the rat is associated with a decrease in GABA receptor-mediated chloride ion flux and brain benzodiazepine receptor occupancy. Brain research. 1989 May 15;487:45–51. doi: 10.1016/0006-8993(89)90938-4. [DOI] [PubMed] [Google Scholar]
- 24.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Molecular psychiatry. 2010 Nov 17;16:383–406. doi: 10.1038/mp.2010.120. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geuze E, van Berckel BN, Lammertsma AA, et al. Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Molecular psychiatry. 2007 Aug 02;13:74–83. 73. doi: 10.1038/sj.mp.4002054. 2008. [DOI] [PubMed] [Google Scholar]
- 26.Vaiva G, Boss V, Ducrocq F, et al. Relationship between posttrauma GABA plasma levels and PTSD at 1-year follow-up. The American journal of psychiatry. 2006 Aug 01;163:1446–1448. doi: 10.1176/ajp.2006.163.8.1446. [DOI] [PubMed] [Google Scholar]
- 27.Gonda X, Sarginson J, Eszlari N, et al. A new stress sensor and risk factor for suicide: the T allele of the functional genetic variant in the GABRA6 gene. Scientific reports. 2017 Oct 12;7:12887. doi: 10.1038/s41598-017-12776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skilbeck KJ, Johnston GA, Hinton T. Stress and GABA receptors. Journal of neurochemistry. 2009 Dec 17;112:1115–1130. doi: 10.1111/j.1471-4159.2009.06539.x. 2010. [DOI] [PubMed] [Google Scholar]
- 29.Akinci MK, Johnston GA. Sex differences in the effects of acute swim stress on binding to GABAA receptors in mouse brain. Journal of neurochemistry. 1993 Jun 01;60:2212–2216. doi: 10.1111/j.1471-4159.1993.tb03507.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilson MA, Biscardi R. Sex differences in GABA/benzodiazepine receptor changes and corticosterone release after acute stress in rats. Experimental brain research. 1994 Jan 01;101:297–306. doi: 10.1007/BF00228750. [DOI] [PubMed] [Google Scholar]
- 31.Hasler G, van der Veen JW, Grillon C, et al. Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. The American journal of psychiatry. 2010 Jul 17;167:1226–1231. doi: 10.1176/appi.ajp.2010.09070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houtepen LC, Schur RR, Wijnen JP, et al. Acute stress effects on GABA and glutamate levels in the prefrontal cortex: A 7T 1H magnetic resonance spectroscopy study. NeuroImage Clinical. 2017 Feb 10;14:195–200. doi: 10.1016/j.nicl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology. 2014 Apr 24;231:3619–3634. doi: 10.1007/s00213-014-3572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen Y, Lenkinski RE. Recent advances in magnetic resonance neurospectroscopy. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2007 Jun 30;4:330–345. doi: 10.1016/j.nurt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prescot AP, Renshaw PF, Yurgelun-Todd DA. gamma-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug and alcohol dependence. 2013 Mar 26;129:232–239. doi: 10.1016/j.drugalcdep.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. The American journal of psychiatry. 2005 Jan 29;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 37.Milak MS, Proper CJ, Mulhern ST, et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Molecular psychiatry. 2015 Aug 19;21:320–327. doi: 10.1038/mp.2015.83. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner G, Koch K, Schachtzabel C, et al. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? NeuroImage. 2010 Sep 14;54:1607–1614. doi: 10.1016/j.neuroimage.2010.08.082. 2011. [DOI] [PubMed] [Google Scholar]
- 39.van Heeringen K, Bijttebier S, Desmyter S, et al. Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Frontiers in human neuroscience. 2014 Nov 07;8:824. doi: 10.3389/fnhum.2014.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews S, Spadoni A, Knox K, et al. Combat-exposed war veterans at risk for suicide show hyperactivation of prefrontal cortex and anterior cingulate during error processing. Psychosomatic medicine. 2012 Apr 19;74:471–475. doi: 10.1097/PSY.0b013e31824f888f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 42.Wechsler D. Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II. San Antonio, TX: NCS Pearson; [Google Scholar]
- 43.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. The American journal of psychiatry. 2011 Dec 24;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960 Feb 01;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton M. The assessment of anxiety states by rating. The British journal of medical psychology. 1959 Jan 01;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 46.JB . Psychological Assessment Resources. 1995. Trauma Symptom Inventory professional manual. [Google Scholar]
- 47.Elhai JD, Gray MJ, Kashdan TB, et al. Which instruments are most commonly used to assess traumatic event exposure and posttraumatic effects?: A survey of traumatic stress professionals. Journal of traumatic stress. 2005 Nov 11;18:541–545. doi: 10.1002/jts.20062. [DOI] [PubMed] [Google Scholar]
- 48.Snyder JJ, Elhai JD, North TC, et al. Reliability and validity of the Trauma Symptom Inventory with veterans evaluated for posttraumatic stress disorder. Psychiatry research. 2009 Nov 13;170:256–261. doi: 10.1016/j.psychres.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Bahraini NH, Brenner LA, Harwood JE, et al. Utility of the trauma symptom inventory for the assessment of post-traumatic stress symptoms in veterans with a history of psychological trauma and/or brain injury. Military medicine. 2009 Nov 07;174:1005–1009. doi: 10.7205/milmed-d-00-9509. [DOI] [PubMed] [Google Scholar]
- 50.McDevitt-Murphy ME, Weathers FW, Adkins JW. The use of the trauma symptom inventory in the assessment of PTSD symptoms. Journal of traumatic stress. 2005 Nov 11;18:63–67. doi: 10.1002/jts.20003. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds WM, Mazza JJ. Reliability and validity of the Reynolds Adolescent Depression Scale with young adolescents. Journal of School Psychology. 1998;36:295–312. DOI: https://doi.org/10.1016/S0022-4405(98)00010-7. [Google Scholar]
- 52.Schulte RF, Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR in biomedicine. 2006 Mar 17;19:255–263. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- 53.Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. Journal of magnetic resonance Series B. 1994 May 01;104:1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- 54.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002 Oct 23;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Ugurbil K, Chen W. Microstrip RF surface coil design for extremely high-field MRI and spectroscopy. Magnetic resonance in medicine. 2001 Sep 11;46:443–450. doi: 10.1002/mrm.1212. [DOI] [PubMed] [Google Scholar]
- 56.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004 Oct 27;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 57.Prescot AP, Richards T, Dager SR, et al. Phase-adjusted echo time (PATE)- averaging 1 H MRS: application for improved glutamine quantification at 2. 89 T. NMR in biomedicine. 2012 Mar 13;25:1245–1252. doi: 10.1002/nbm.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prescot AP, Renshaw PF. Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (ProFit) in the frontal and parietal lobes of healthy volunteers: assessment of metabolite discrimination and general reproducibility. Journal of magnetic resonance imaging : JMRI. 2012 Oct 12;37:642–651. doi: 10.1002/jmri.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013 May 16;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jollant F, Richard-Devantoy S, Ding Y, et al. Prefrontal inositol levels and implicit decision-making in healthy individuals and depressed patients. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2016 Jun 28;26:1255–1263. doi: 10.1016/j.euroneuro.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Gabbay V, Bradley KA, Mao X, et al. Anterior cingulate cortex gamma-aminobutyric acid deficits in youth with depression. Translational psychiatry. 2017 Sep 12;7:e1216. doi: 10.1038/tp.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013 Feb 12;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Jollant F, Near J, Turecki G, et al. Spectroscopy markers of suicidal risk and mental pain in depressed patients. Progress in neuro-psychopharmacology & biological psychiatry. 2016 Dec 17; doi: 10.1016/j.pnpbp.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Kuang WH, Zou K, et al. A proton magnetic spectroscopy research on hippocampus metabolisms in people with suicide-attempted depressions. Sichuan da xue xue bao Yi xue ban = Journal of Sichuan University Medical science edition. 2009 Mar 19;40:59–62. [PubMed] [Google Scholar]
- 65.De Stefano N, Narayanan S, Francis SJ, et al. Diffuse axonal and tissue injury in patients with multiple sclerosis with low cerebral lesion load and no disability. Archives of neurology. 2002 Oct 11;59:1565–1571. doi: 10.1001/archneur.59.10.1565. [DOI] [PubMed] [Google Scholar]
- 66.Matthews PM, De Stefano N, Narayanan S, et al. Putting magnetic resonance spectroscopy studies in context: axonal damage and disability in multiple sclerosis. Seminars in neurology. 1998 Nov 17;18:327–336. doi: 10.1055/s-2008-1040884. [DOI] [PubMed] [Google Scholar]
- 67.Rumpel H, Khoo JB, Chang HM, et al. Correlation of the apparent diffusion coefficient and the creatine level in early ischemic stroke: a comparison of different patterns by magnetic resonance. Journal of magnetic resonance imaging : JMRI. 2001 Mar 10;13:335–343. doi: 10.1002/jmri.1048. [DOI] [PubMed] [Google Scholar]
- 68.Taylor JM, Whalen PJ. Neuroimaging and Anxiety: the Neural Substrates of Pathological and Non-pathological Anxiety. Current psychiatry reports. 2015 May 10;17:49. doi: 10.1007/s11920-015-0586-9. [DOI] [PubMed] [Google Scholar]
- 69.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain : a journal of neurology. 1995 Feb 01;118( Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 70.Baldacara L, Zugman A, Araujo C, et al. Reduction of anterior cingulate in adults with urban violence-related PTSD. Journal of affective disorders. 2014 Jul 18;168:13–20. doi: 10.1016/j.jad.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 71.Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Archives of general psychiatry. 1999 Nov 24;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 72.Lu YR, Fu XY, Shi LG, et al. Decreased plasma neuroactive amino acids and increased nitric oxide levels in melancholic major depressive disorder. BMC psychiatry. 2014 Apr 29;14:123. doi: 10.1186/1471-244x-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roy A, Dejong J, Ferraro T. CSF GABA in depressed patients and normal controls. Psychological medicine. 1991 Aug 01;21:613–618. doi: 10.1017/s0033291700022248. [DOI] [PubMed] [Google Scholar]
- 74.Price RB, Shungu DC, Mao X, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biological psychiatry. 2008 Dec 09;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brennan BP, Admon R, Perriello C, et al. Acute change in anterior cingulate cortex GABA, but not glutamine/glutamate, mediates antidepressant response to citalopram. Psychiatry research. 2017 Sep 12;269:9–16. doi: 10.1016/j.pscychresns.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levey DF, Niculescu EM, Le-Niculescu H, et al. Towards understanding and predicting suicidality in women: biomarkers and clinical risk assessment. Molecular psychiatry. 2016 Apr 06;21:768–785. doi: 10.1038/mp.2016.31. [DOI] [PubMed] [Google Scholar]
- 77.Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Archives of general psychiatry. 2002 Sep 07;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 78.Schur RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Human brain mapping. 2016 May 05;37:3337–3352. doi: 10.1002/hbm.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2011 Sep 06;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. 2012. [DOI] [PubMed] [Google Scholar]
- 80.Kelmendi B, Adams TG, Yarnell S, et al. PTSD: from neurobiology to pharmacological treatments. European journal of psychotraumatology. 2016 Nov 13;7:31858. doi: 10.3402/ejpt.v7.31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.