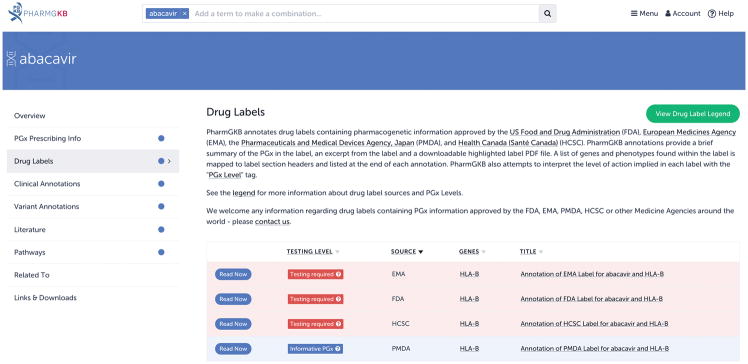
Figure 3.
An overview of the annotated drug labels for abacavir on PharmGKB. PharmGKB annotates drug labels approved by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), Health Canada (HCSC) and the Pharmaceutical and Medical Devices Agency, Japan (PMDA). PharmGKB has annotated drug labels available for over 250 drugs; not every drug has annotated labels available from all four of these medicine agencies.