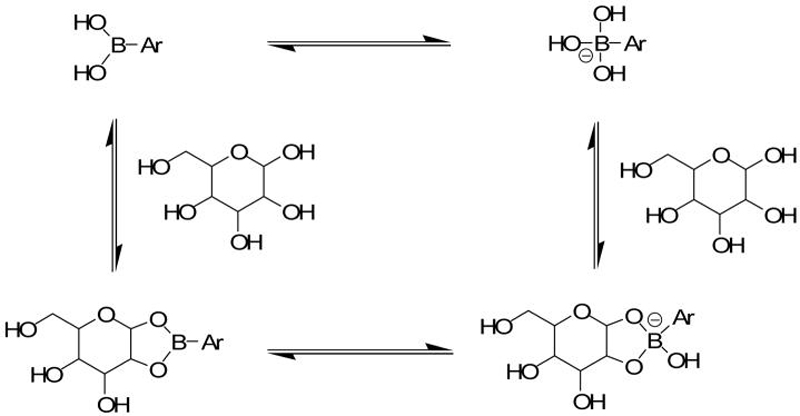
Figure 11.
Saccharide-induced hybridization and concomitant polarization changes. When saccharides form cyclic boronates, the Lewis acidity of the boronic acid is enhanced by at least ~2 pKa units, resulting in a solvolysis reaction at boron. Thus, the sp2 hybridized boronic acid is more readily converted to a charged sp3 hybridized anion upon saccharide binding. In most cases, cyclic boronate ester formation occurs with a sugar furanose (rather than the shown pyranose).