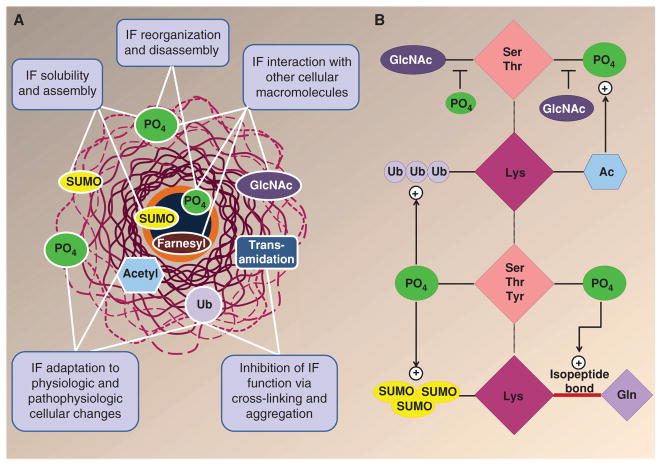
Figure 4.
Posttranslational modifications (PTMs) of intermediate filament (IF) proteins. (A) IFs undergo multiple PTMs, including phosphorylation (PO4), O-linked glycosylation (GlcNAc), ubiquitination (Ub), acetylation (acetyl), SUMOylation (SUMO), transamidation, and farnesylation (Snider and Omary 2014). Keratins also undergo all these modifications except for farnesylation, which specifically occurs in lamin IF proteins. Multiple functions are ascribed to IF/keratin PTMs, as highlighted in the panel text boxes. (B) Keratin PTMs target residues that can be modified by multiple PTMs. For example, Ser/Thr residues can be phosphorylated and glycosylated, whereas Lys residues can be modified by acetylation, ubiquitination, SUMOylation, and transamidation. There is also cross talk between different modifications, as exemplified by the effect of phosphorylation on several PTMs, and vice versa. The multiple combinations of modifications on a given keratin can provide a complex hierarchy of regulation. (Modified from Snider and Omary 2014.)