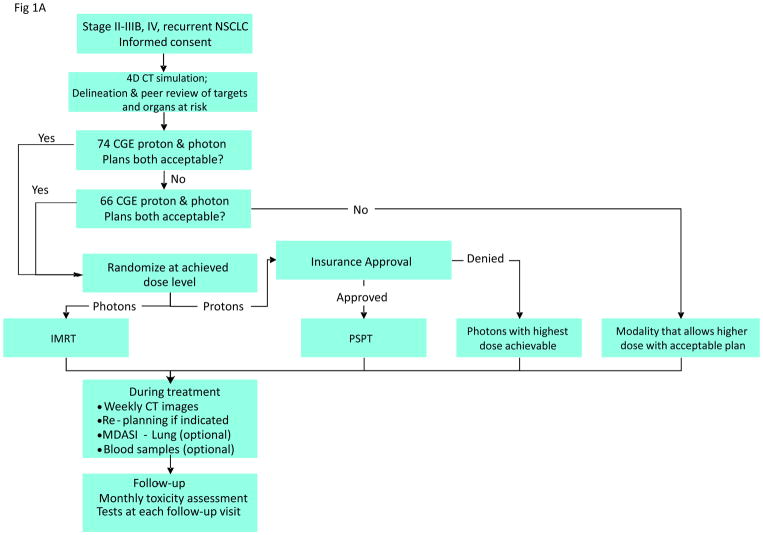
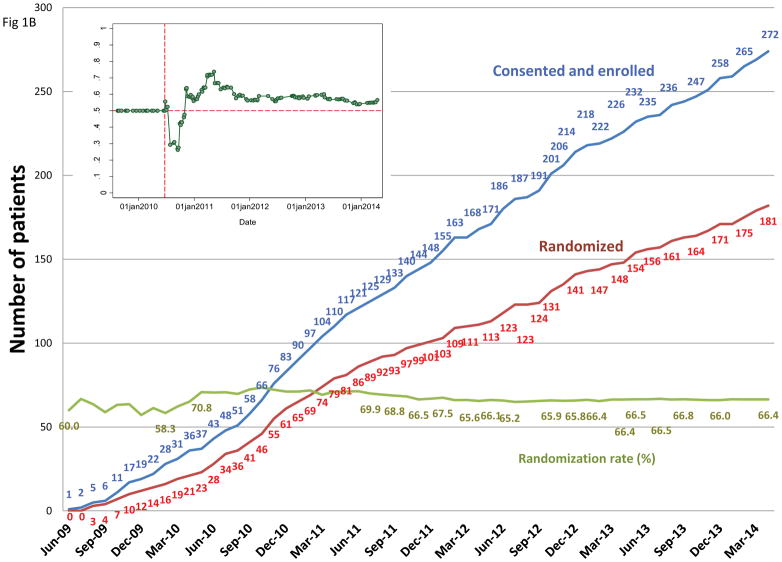
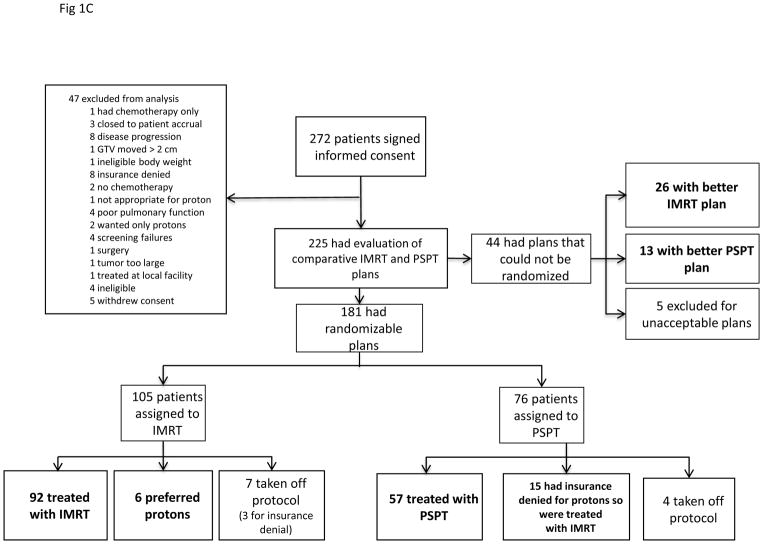
Figure 1.
A, The adaptive-randomization process. Eligibility criteria included stage II–IIIB non-small cell lung cancer (NSCLC) or stage IV NSCLC with a single brain metastasis or isolated tumor recurrence after surgical resection; 50% of patients had disease progression after systemic chemotherapy before enrollment. All patients underwent four-dimensional computed tomography (4D CT) -based treatment simulation, and target volume contours and preliminary plans were reviewed before randomization. The prescribed dose for both sets of treatment plans (intensity-modulated [photon] radiation therapy [IMRT] and passive scattered proton therapy [PSPT]) was 74 Gy(RBE). If one of the two plans did not meet prespecified dose constraints (Suppl Table S1), the prescribed dose was reduced to 66 Gy for a second pair of plans. Patients were randomized only when both IMRT and PSPT plans met the dose-constraint standards. If one of the two plans did not meet dose constraints at the 66-Gy(RBE) level, the patient was treated with the modality that produced the acceptable dose distribution. During treatment, weekly 4D CT scans were obtained for all patients and additional treatment plans were created as needed to account for anatomic changes during treatment. To ensure that the radiation pneumonitis events (“toxicity assessment”) was noted accurately, patients were contacted weekly with a questionnaire to assess symptoms of pneumonitis. B, Cumulative patient randomization (red) and enrollment (blue) over time. Inset, posterior probability of randomization to IMRT, with the vertical dashed line representing the last date (June 22, 2010) at which the randomization probability was 0.5. The first patient was randomized on August 17, 2009 and the last patient on April 18, 2014; 60% to 67% of all patients who consented to participate were eligible for randomization. C, Trial profile. The final numbers of patients included in the analysis are shown in bold.