Figure 2. Analysis of day 2 serum and tissue viral titers in RVFV-infected hamsters treated with galidesivir (efficacy experiment 1).
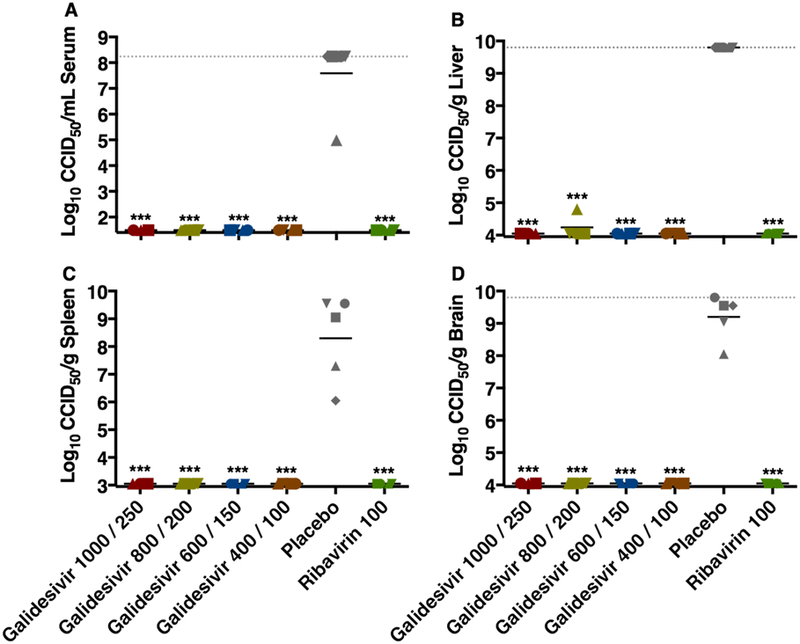
Hamsters were treated as described in Table 1 and Figure 1. Four (5 for placebo) animals in each group were designated for sacrifice on day 2 p.i. for analysis of A) serum, B) liver, C) spleen, and D) brain virus titers. Unique symbols in each treatment group represent values for the same animal across all parameters. The x-axis represents the lower limit of detection while the grey-hashed lines indicate the assay upper limits of detection. One animal receiving the highest dose of galidesivir succumbed prior to sacrifice, and thus is not included in the analysis. ***P < 0.001 compared to animals receiving placebo.
