Fig 2.
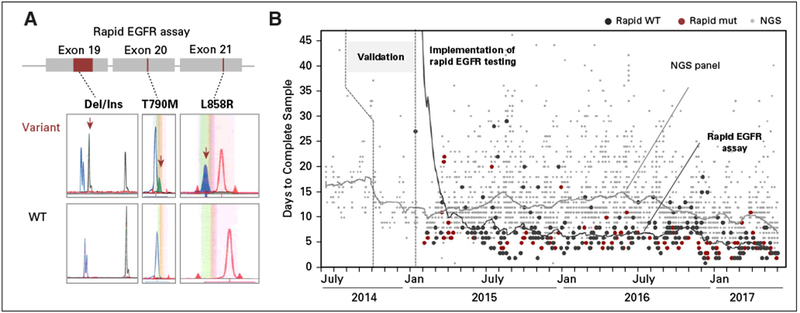
Rapid EGFR assay and turnaround times compared with next-generation sequencing (NGS)–based genotyping. (A) The rapid EGFR assay consists of three separate reactions: a sizing assay to identify exon 19 (ELREA sequence) deletions and two single-nucleotide extension reactions to identify p.T790M and p.L858R missense mutations. (B) After validation (last quarter of 2014), we implemented rapid EGFR genotyping in January 2015. Scatter plots portray turnaround times of all 243 rapid EGFR samples (Jan 2015 to May 2017; black, EGFR wild type [WT]; red, EGFR mutation [mut] detected) and all specimens that underwent NGS (gray dots) during this period. Note that process improvements have led to a reduction in average turnaround times for both assays (lines).
