Abstract
Objective:
To evaluate the efficacy and safety of mealtime or post-meal fast-acting insulin aspart (faster aspart) versus mealtime insulin aspart (IAsp), both in combination with insulin degludec, in participants with T1D.
Research Design and Methods:
This multicentre, treat-to-target trial randomized participants to double-blind mealtime faster aspart (n=342) or IAsp (n=342), or open-label post-meal faster aspart (n=341). The primary endpoint was change from baseline in HbA1c 26 weeks post-randomization. All available information regardless of treatment discontinuation was used for evaluation of the effect.
Results:
Non-inferiority for the change from baseline in HbA1c was confirmed for mealtime and post-meal faster aspart versus IAsp (estimated treatment difference [ETD 95%CI] −0.02% [-0.11;0.07] and 0.10% [0.004;0.19], respectively). Mealtime faster aspart was superior to IAsp for 1-h PPG increment using a meal test (ETD −0.90 mmol/L [−1.36;−0.45]; P<0.001). Self-monitored 1-h PPG increment favoured faster aspart at breakfast (ETD −0.58 mmol/L [−0.99;−0.17]; P=0.006) and across all meals (−0.48 mmol/L [-0.74;−0.21]; P<0.001). Safety profiles and overall rate of severe or blood glucose-confirmed hypoglycaemia were similar between treatments, but significantly less hypoglycaemia was seen 3–4 h after meals with mealtime faster aspart.
Conclusion:
Mealtime and post-meal faster aspart with insulin degludec provided effective glycaemic control compared with IAsp with no increased safety risk. Mealtime faster aspart provided superior PPG control to IAsp.
Introduction
To reduce the incidence and slow the progression of diabetes-related complications, guidelines recommend HbA1c target levels.1–6 Achievement of the conventionally accepted HbA1c target <7.0% (53 mmol/mol) in people with type 1 diabetes (T1D) usually requires postprandial glycaemic control.4–6
Exogenous mealtime insulin administration aims to mimic the physiological secretion pattern of insulin to control postprandial glucose (PPG) excursions. There is an unmet need for mealtime insulin that better mimics physiological control, while enhancing flexibility and treatment convenience for patients. The development in basal insulin analogues, including ultra-long-acting and high-concentration insulins such as insulin degludec (degludec) and glargine U300,7 is now being complemented by emergence of ultra-fast-acting mealtime insulins. These agents incorporate modifications to insulin formulations to accelerate absorption and thereby improve insulin time-action and PPG-lowering profiles.8–12
Fast-acting insulin aspart (faster aspart) is a faster-acting mealtime insulin with a more rapid rate of absorption into the bloodstream and greater early-glucose-lowering effect than conventional insulin aspart (IAsp).13 Faster aspart has been approved for use by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) as treatment for diabetes in adults.
In the onset 1 trial, efficacy and safety of faster aspart were evaluated as part of a basal-bolus regimen with insulin detemir (detemir) in participants with T1D.14 A statistically significant yet modest improvement in HbA1c was observed with mealtime faster aspart compared with IAsp after 26 weeks’ treatment (estimated treatment difference [ETD] −0.15% [95%CI −0.23;−0.07]; −1.62 mmol/mol [−2.50;−0.73]), with a superior reduction in 2-h PPG increment during a standardized meal test.14 This improvement in glycaemic control was maintained after 52 weeks’ treatment.15 Faster aspart dosed 20 min after the start of meal was non-inferior (0.4% margin) to mealtime IAsp regarding change in HbA1c after 26 weeks’ treatment.14
To date, no studies are available comparing faster aspart with IAsp in a basal-bolus regimen with ultra-long-acting basal insulins. The onset 8 study is similar in design to onset 1, and aims to evaluate the efficacy and safety of faster aspart in conjunction with degludec in participants with T1D. Onset 8 was designed to quantify a population average effect, irrespective of adherence to randomized treatment and ancillary therapy use. The primary objective was to estimate the effect based on difference in HbA1c from baseline to 26 weeks, under these circumstances. As it can sometimes be challenging to administer bolus insulin before a meal, a post-meal faster aspart arm (within 20 min after start of meal) was also included to further evaluate the option of dosing faster aspart after a meal when needed.
Research design and methods
Trial design
In this phase 3b, multicentre, active-controlled, randomized, parallel-group study (ClinicalTrials.gov: NCT02500706), mealtime faster aspart was compared with mealtime IAsp, both double-blind, in adults with T1D, over 26 weeks (Figure S1). In a third, open-label treatment arm, participants received post-meal faster aspart (Figure S1). Faster aspart and IAsp were delivered in a basal-bolus regimen with once-daily degludec. The trial was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization of Good Clinical Practice. The study was conducted at 146 centres in 12 countries/regions (Supplementary Appendix). Follow-up occurred 7 and 30 days after end of treatment.
Study population
Adults (≥18 years old; ≥20 years in Japan, Taiwan) with T1D were eligible for inclusion if they received a basal-bolus insulin regimen for ≥12 months before screening, including a basal insulin analogue for ≥4 months before screening. Eligibility also required HbA1c 7.0–9.5% (53–80 mmol/mol) and BMI ≤35.0 kg/m2.
Key exclusion criteria included: treatment with any antidiabetic or obesity medication (other than listed inclusion criteria) within 3 months before screening; any anticipated change in concomitant medications known to affect glucose metabolism persisting beyond 2 weeks of study inclusion; myocardial infarction, stroke or hospitalization for unstable angina and/or transient ischaemic attack within 180 days before screening; use of continuous glucose monitoring; inadequately-treated hypertension (≥Class 2) or clinically significant hepatic or renal insufficiency (full criteria in Supplementary Appendix).
Interventions
Basal titration during the trial
After a 2-week screening period, an 8-week run-in allowed for basal insulin titration, and participants switched from their existing basal insulin analogue to degludec (100 U/mL, 3-mL pen injector) at the start, based on protocol-specified guidelines (Table S1). During run-in, the investigator titrated degludec on a weekly basis to the pre-breakfast glycaemic target of 4.0–5.0 mmol/L in accordance with titration guidelines (Table S2). Further adjustment of degludec during the treatment period was performed at investigator’s discretion.
Bolus dosing during the trial
At start of run-in, participants switched from their previous bolus insulin to IAsp (all bolus insulins supplied as 100 U/mL, 3-mL pen injector) on a unit-for-unit basis. IAsp dose was not adjusted during run-in unless considered necessary by the investigator. At end of run-in, participants with HbA1c ≤9.5% (80 mmol/mol) were randomized 1:1:1 to receive double-blind mealtime IAsp, double-blind mealtime faster aspart, or open-label post-meal faster aspart. Mealtime insulins were injected 0–2 min before a meal, while post-meal faster aspart was injected at the end (no later than 20 min after start of meal).
During the 26-week treatment period, bolus insulin was titrated to achieve a glycaemic target of pre-prandial and bedtime plasma glucose (PG) between 4.0 and 6.0 mmol/L. Participants considered proficient in carbohydrate counting continued using this method for bolus adjustment during the treatment period, while all other participants used a predefined bolus-dosing algorithm (Table S3).
Meal carbohydrate content and pre-prandial BG values were used to determine bolus-insulin doses for participants based on flexible dosing principles. Doses were calculated several times daily by each participant based on the insulin:carbohydrate ratio and insulin correction factor. Weekly review of the ratio and correction factor was performed by the investigator, based on each participant’s self-measured blood glucose (SMBG) values. In the event of hypoglycaemia, the dose could be reduced at investigator’s discretion.
Self-measured blood glucose (SMBG)
Participants were supplied with a BG meter (Abbott Precision Neo or Precision; Abbott Laboratories, Chicago, USA), and were instructed to record date, time, and value of all SMBG measurements relating to 4-, 7-, and 9-point profiles and hypoglycaemic episodes. 4-point profiles (before each main meal [breakfast, lunch, evening meal], and bedtime) were recorded daily for insulin titration purposes. 7–9-7-point profiles (before and 60 min after each main meal, at bedtime, at 04:00 h [9-point only], before breakfast next day [9-point only]) were recorded on three consecutive days (7-point profiles for days 1 and 3, a 9-point profile on day 2) before scheduled clinic visits at weeks 0, 12, and 26.
Standardized meal test
Participants were required to attend the test with a fasting SMBG of 4.0–8.8 mmoL/L. Before randomization at week 0 (baseline), a bolus dose of IAsp was administered (0.1 U/kg, calculated by investigator), followed by a standardized mixed liquid-meal test (78 g carbohydrate consumed within 12 min). Blood samples were taken immediately before the meal, and after 30 min, 1, 2, 3, and 4 h (0 h defined as start time of meal consumption). The meal test was repeated at week 26 using the participant’s study medication. Participants randomized to post-meal insulin received their bolus dose 20 min after starting the meal.
Assessments
Primary endpoint
The primary endpoint was change from baseline in HbA1c after 26 weeks’ treatment.
Secondary endpoints
Confirmatory secondary endpoints were change from baseline in 1-h PPG increment (meal test) and change from baseline in 1,5-anhydroglucitol (1,5-AG), both at week 26.
Supportive secondary efficacy endpoints were: change from baseline in fasting plasma glucose (FPG); participants (%) reaching HbA1c targets (<7.0% [53 mmol/mol]); change from baseline in 30-min, 1-h, 2-h, 3-h and 4-h PPG; change from baseline in 30-min, 2-h, 3-h and 4-h PPG increment (meal test); change from baseline in 7–9-7-point SMBG assessed by the mean of the 7–9-7-point profile, PPG and PPG increment (at each meal and over all meals); percentage of participants reaching 1-h PPG targets (≤7.8 mmol/L); change from baseline in lipid-lipoprotein profiles; basal, total, and individual meal insulin doses (Table S4).
Supportive secondary safety endpoints were: treatment-emergent adverse events (TEAEs), treatment-emergent injection site reactions; treatment-emergent hypoglycaemic episodes (overall, and after meal [1, 1–2, 2–3 and 3–4 h timepoints]); physical examination; vital signs; electrocardiogram; fundoscopy; laboratory parameters; anti-IAsp antibody development (specific and cross-reacting with human insulin); and body weight. (See Supplementary Appendix for TEAE and treatment-emergent hypoglycaemia definitions.)
Statistical methods
All statistical analyses were prespecified. Efficacy endpoints were summarized using the full analysis set, and results are presented based on data from all randomized participants for the entire trial period, which includes data collected after participants prematurely discontinued treatment (in-trial observation period). Safety endpoints were summarized using the safety analysis set (participants receiving ≥1 dose of IAsp or faster aspart). Statistical analysis of primary and secondary confirmatory endpoints followed a stepwise hierarchical procedure, which was stopped after step 4 (Figure S2, Table S5). Non-inferiority (primary endpoint) was confirmed if the upper boundary of the two-sided 95%CI was ≤0.4%. Two-sided P-values are presented unless stated otherwise.
Change from baseline in HbA1c 26 weeks post-randomization was analysed using a statistical model using multiple imputations where participants without any available HbA1c measurements at scheduled visits had their HbA1c value imputed from the available information from the treatment arm to which the participant had been randomized. Change from baseline in PPG and PPG increment (meal test) was analysed using an analysis of variance model, and HbA1c and PPG responder endpoints were analysed using a logistic regression model. Change from baseline in 7–9-7-point outcomes (mean SMBG, PPG, PPG increments), 1,5-AG, FPG, lipid-lipoprotein profiles (log-transformed) and body weight were analysed using a model similar to the statistical model for the primary endpoint. Number of treatment-emergent severe or BG-confirmed hypoglycaemic episodes was analysed using a negative binomial regression model.
Sample-size calculation and further details on statistical methods for the primary and secondary endpoints are in the Supplementary Appendix.
Results
Trial participants
In total, 1025 participants were randomized to mealtime faster aspart (n=342), IAsp (n=342), or post-meal faster aspart (n=341), and all were exposed to their study medication. 1007 participants (98.2%) completed the trial, while 999 participants (97.5%) similarly distributed across treatment arms completed the 26-week treatment period without premature discontinuation of randomized treatment (Figure S3). The most common reason for withdrawal from the trial was ‘withdrawal by subject’ (4 participants in both the mealtime faster aspart and IAsp arms, 6 in post-meal faster aspart arm). Premature discontinuation of randomized treatment occurred in 7, 9, and 10 participants in the mealtime faster aspart, post-meal faster aspart and mealtime IAsp arms, respectively. Baseline demographic and disease characteristics were similar between the three treatment arms (Table 1).
Table 1.
Baseline characteristics
| Parameter | Faster aspart (mealtime)(n=342) | Faster aspart (post-meal)(n=341) | Insulin aspart (mealtime)(n=342) | Total(n=1025) |
|---|---|---|---|---|
| Age, years | 41.5 (14.4) | 41.0 (14.6) | 40.8 (14.2) | 41.1 (14.4) |
| Gender, n (% male) | 184 (53.8) | 186 (54.5) | 179 (52.3) | 549 (53.6) |
| Body weight, kg | 72.6 (16.6) | 71.9 (16.9) | 71.8 (17.0) | 72.1 (16.8) |
| BMI, kg/m2 | 25.1 (4.1) | 25.1 (4.4) | 25.1 (4.4) | 25.1 (4.3) |
| Duration of diabetes, years | 17.6 (12.5) | 15.8 (10.6) | 16.7 (11.0) | 16.7 (11.4) |
| HbA1c, % mmol/mol) | 7.5 (0.7)58.0 (7.5) | 7.4 (0.6)57.4 (6.6) | 7.4 (0.8)57.5 (8.7) | 7.4 (0.7)57.7 (7.6) |
| FPG, mmol/L | 6.8 (2.1) | 6.9 (2.5) | 6.8 (2.5) | 6.8 (2.4) |
| Bolus adjusting method, n (% carbohydrate counting) | 142 (41.5) | 150 (44.0) | 136 (39.8) | 428 (41.8) |
Data are presented as means (SD) unless otherwise stated. Faster aspart, fast-acting insulin aspart; FPG, fasting plasma glucose; SD, standard deviation.
Efficacy
Change in HbA1c
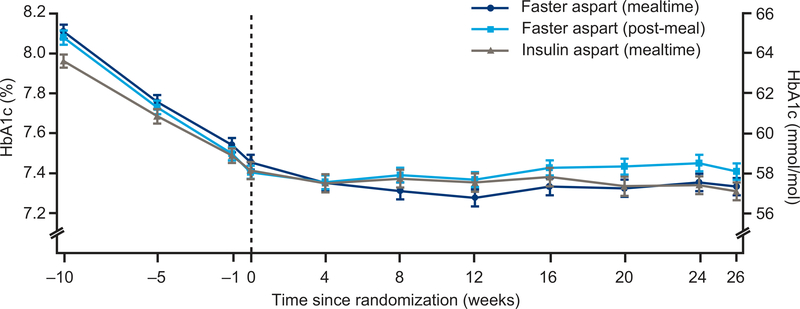
During the run-in period, observed mean HbA1c was reduced from 8.1% (65 mmol/mol) to 7.5% (58 mmol/mol) for participants subsequently randomized to mealtime faster aspart; from 8.0% (63 mmol/mol) to 7.4% (57 mmol/mol) for those randomized to mealtime IAsp; and from 8.1% (65 mmol/mol) to 7.4% (57 mmol/mol) for those randomized to post-meal faster aspart (Figure 1). At the end of the 26-week treatment period, mean HbA1c was 7.3% (57 mmol/mol), 7.3% (56 mmol/mol), and 7.4% (57 mmol/mol) in the mealtime faster aspart, mealtime IAsp, and post-meal faster aspart arms, respectively. Non-inferiority of both mealtime and post-meal faster aspart to mealtime IAsp regarding change from baseline in HbA1c was confirmed (ETD [95%CI] mealtime −0.02% [−0.11;0.07], −0.24 mmol/mol [−1.24;0.76]; post-meal 0.10% [0.004;0.19]; 1.04 mmol/mol [0.04;2.04]; one-sided P<0.001 for non-inferiority). Superiority of mealtime faster aspart versus IAsp regarding change from baseline in HbA1c could not be confirmed (hierarchical testing stopped; Table S5).
Figure 1. Mean HbA1c over time.
During the run-in period, observed mean HbA1c was reduced from 8.1% (65 mmol/mol) to 7.5% (58 mmol/mol) for participants subsequently randomized to mealtime faster aspart; from 8.0% (63 mmol/mol) to 7.4% (57 mmol/mol) for those randomized to mealtime insulin aspart; and from 8.1% (65 mmol/mol) to 7.4% (57 mmol/mol) for those randomized to post-meal faster aspart. At the end of the 26-week treatment period, mean HbA1c was 7.3% (57 mmol/mol), 7.3% (56 mmol/mol), and 7.4% (57 mmol/mol) in the mealtime faster aspart, mealtime insulin aspart, and post-meal faster aspart arms, respectively.
Error bars: ± standard error. All available information regardless of treatment discontinuation was used.
Faster aspart, fast-acting insulin aspart.
The odds of achieving HbA1c <7.0% (53 mmol/mol) were not statistically significantly different between mealtime faster aspart and IAsp, or between post-meal faster aspart and mealtime IAsp (Table S6).
Meal test
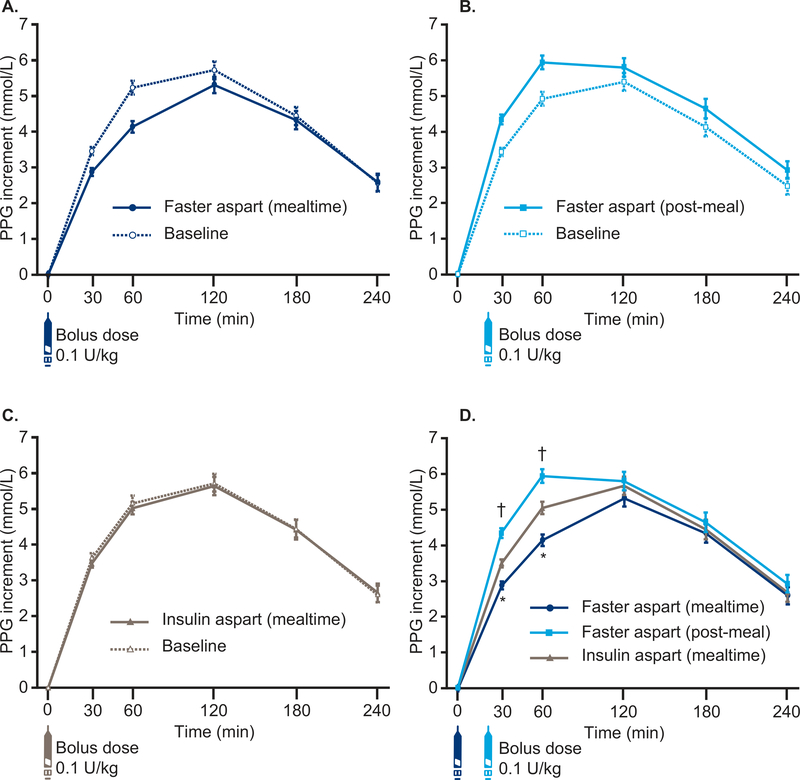
Superiority of mealtime faster aspart to IAsp regarding change from baseline in 1-h PPG increment was confirmed (ETD [95%CI] −0.90 mmol/L [−1.36;−0.45]; P<0.001) (Figure 2D). Change from baseline in 30-min PPG increment was also significantly in favour of mealtime faster aspart; however, there was no statistical significance at other timepoints (Table S6). For the post-meal comparison, change from baseline in 30-min and 1-h PPG increment was significantly in favour of IAsp, with no statistical differences at other timepoints (Figure 2D, Table S6). PPG results (without adjustment for pre-prandial PG) also favoured mealtime faster aspart at 30 min and 1 h with ETDs [95%CI] of −0.82 mmol/L [-1.28;−0.36; P=0.001] and −1.24 mmol/L [95%CI −1.81;−0.67; P<0.001], respectively, and no further statistical differences were observed at other timepoints.
Figure 2. PPG increment after a standardized meal test at week 26.
A) Mealtime faster aspart at week 26 vs baseline; B) Post-meal faster aspart at week 26 vs baseline. C) Mealtime insulin aspart at week 26 vs baseline; D) Mealtime faster aspart, post-meal faster aspart and mealtime insulin aspart at week 26. *P<0.001 in favour of mealtime faster aspart vs insulin aspart; †P<0.001 in favour of insulin aspart vs post-meal faster aspart.
Mealtime insulin aspart dosed immediately before the liquid meal; post-meal faster aspart dosed 20 min after the start of the liquid meal.
Error bars: ± standard error.
All available information regardless of treatment discontinuation was used.
Faster aspart, fast-acting insulin aspart; PPG, postprandial glucose.
SMBG
Observed mean 9-point SMBG profiles at baseline and after 26 weeks’ treatment were similar between groups (Figure S4), and there were no statistically significant differences in mean SMBG for mealtime or post-meal comparisons (Table S6). Regarding change from baseline in 1-h PPG increment (based on SMBG), ETDs [95%CI] were statistically in favour of mealtime faster aspart at breakfast (−0.58 mmol/L [−0.99;−0.17]; P=0.006) and over all meals (−0.48 mmol/L [−0.74;−0.21]; P<0.001), and no differences were reported for the faster aspart post-meal comparison. Change from baseline in 1-h PPG, based on SMBG profiles, for any individual meal (breakfast, lunch, evening meal) or for ‘all meals’ was not statistically significantly different for mealtime faster aspart versus IAsp (Table S6). However, for the faster aspart post-meal comparison, change from baseline in 1-h PPG for ‘all meals’ was statistically significant in favour of IAsp (ETD [95%CI] 0.34 mmol/L [0.06;0.63]).
The percentage of participants who achieved 1-h PPG ≤7.8 mmol/L was significantly greater with mealtime faster aspart (27.8%) than with IAsp (21.6%; estimated odds ratio 1.54 [95%CI 1.05;2.26]; P=0.028). There was no statistically significant difference for the faster aspart post-meal comparison.
Other secondary endpoints
After 26 weeks there was a numerical increase in 1,5-AG in the mealtime faster aspart and IAsp arms, with no significant difference between the two arms (ETD [95%CI] 0.02 μg/mL [-0.31;0.34]). In the post-meal faster aspart arm there was a slight decrease in 1,5-AG, which was statistically significant versus mealtime IAsp (−0.35 μg/mL [−0.68;−0.03]; P=0.035).
In all three treatment arms, mean FPG showed an increase from baseline to week 12 and a decrease thereafter, to week 26. Fasting SMBG levels on days when meal tests were performed had to be 4.0–8.8 mmol/L. At week 12, there was no such requirement. The estimated difference in FPG between mealtime faster aspart and IAsp at week 26 was statistically significant (ETD [95%CI] −0.39 mmol/L [−0.78;−0.0008]; P=0.05). There was no significant difference between post-meal faster aspart and mealtime IAsp (Table S6). No clinically significant differences were seen in lipid-lipoprotein profiles.
Safety
Hypoglycaemia results are presented in Table 2. No statistically significant differences were observed between the faster aspart (mealtime or post-meal) and IAsp arms regarding the rate of treatment-emergent severe or BG-confirmed hypoglycaemic episodes, and the rate of treatment-emergent severe or BG-confirmed hypoglycaemic episodes during the first, second, or third hour after start of meal. However, a significant difference in the rate of severe or BG-confirmed hypoglycaemic episodes occurring 3–4 h after start of meal was observed, in favour of mealtime faster aspart versus IAsp (0.72 [0.54;0.96]; P=0.024). No statistically significant difference between treatment groups was observed for mean body weight at week 26 (change from baseline: +1.43 kg [mealtime faster aspart], +1.14 kg [post-meal faster aspart], +1.24 kg [insulin aspart]).
Table 2.
Treatment-emergent hypoglycaemic events
| Faster aspart (mealtime) | Faster aspart (post-meal) | Insulin aspart (mealtime) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | E | R | N | % | E | R | N | % | E | R | |
| Treatment-emergent hypoglycaemia* | ||||||||||||
| Severe | 32 | 9.4 | 46 | 0.27 | 19 | 5.6 | 29 | 0.17 | 31 | 9.1 | 47 | 0.28 |
| Severe or BG-confirmed | 304 | 88.9 | 5839 | 34.09 | 295 | 86.5 | 6707 | 39.40 | 302 | 88.3 | 6820 | 40.08 |
| Meal-related severe or BG-confirmed hypoglycaemia* | ||||||||||||
| Within 1 h after a meal | 84 | 24.6 | 255 | 1.49 | 88 | 25.8 | 265 | 1.56 | 88 | 25.7 | 217 | 1.28 |
| Between 1 and 2 h after a meal | 111 | 32.5 | 300 | 1.75 | 110 | 32.3 | 443 | 2.60 | 111 | 32.5 | 407 | 2.39 |
| Between 2 and 3 h after a meal | 135 | 39.5 | 512 | 2.99 | 160 | 46.9 | 745 | 4.38 | 156 | 45.6 | 609 | 3.58 |
| Between 3 and 4 h after a meal | 149 | 43.6 | 488 | 2.85 | 165 | 48.4 | 684 | 4.02 | 156 | 45.6 | 681 | 4.00 |
Hypoglycaemia was defined as treatment-emergent if the onset of the episode occurred on or after the first day of treatment administration post-randomization and no later than one day after the last day on treatment. Severe hypoglycaemia was defined according to the American Diabetes Association classification,23 and BG-confirmed hypoglycaemia was defined as a plasma glucose value <3.1 mmol/L (Novo Nordisk A/S definition) with or without symptoms consistent with hypoglycaemia.
%, percentage of participants; BG, blood glucose; E, number of events; faster aspart, fast-acting insulin aspart; N, number of participants; R, event rate per patient-year of exposure.
No clinically significant differences between treatment groups were observed regarding percentage of participants reporting TEAEs and the overall rate of TEAEs (similar between the three treatment arms; Table S8); injection-site and allergic reactions, which were low in number and evenly distributed across the three treatment arms (Table S8); vital signs, physical examination and safety laboratory assessments. Additional details are shown in the Supplementary Appendix.
During the trial, mean and median daily bolus insulin doses increased by similar amounts in all three treatment groups (Table S7), and the basal/bolus ratio 26 weeks post-randomization had changed to a higher proportion of bolus compared with basal insulin across the three treatment arms (Table S7).
Discussion
The results of this study confirm that both mealtime and post-meal faster aspart, in combination with degludec, were non-inferior to mealtime IAsp regarding change in HbA1c from baseline to 26 weeks in people with T1D. Furthermore, mealtime faster aspart was effective in reducing PPG excursions, and superiority to IAsp in 1-h PPG increment (following a standardized meal test) was confirmed. Mean SMBG-derived 1-h PPG increments also showed a trend towards improved PPG control, with mealtime faster aspart conferring a significantly lower 1-h PPG increment than IAsp at breakfast and also when averaged over all meals. Furthermore, significantly more participants receiving mealtime faster aspart achieved a 1-h PPG target <7.8 mmol/L (based on SMBG) compared with IAsp. Change in body weight was <2 kg in all three treatment arms, which may be expected given that participants were already on a basal-bolus regimen at baseline.
The improved PPG findings (both meal test and SMBG) reported in the present study are consistent with the general findings across the faster aspart clinical trial programme.14–17 Furthermore, the results here specifically support the results of the onset 1 study, where once- or twice-daily detemir with mealtime or post-meal faster aspart were compared with the same basal regimen and mealtime IAsp.14 In onset 1, however, there was a statistically significant difference in HbA1c change after 26 weeks between mealtime faster aspart and IAsp, unlike in the present study. Despite the reported improvements in PPG and similar patient populations in both studies, it is unclear why a greater difference in change in HbA1c between mealtime faster aspart and IAsp was not observed in the present study. There was also a discrepancy between the two studies in the incidence of meal-related hypoglycaemia at 1 h after the start of a main meal. In the present study, there was no significant difference between mealtime faster aspart and IAsp regarding the rate of severe or BG-confirmed hypoglycaemic episodes 1 h after a main meal, whereas a statistically significant difference in favour of IAsp was recorded in onset 1 (rate ratio: faster aspart/IAsp 1.48 [95%CI 1.11;1.96]; P=0.0073).14 Conversely, there was a significant reduction in meal-related hypoglycaemia in favour of mealtime faster aspart 3–4 h after a main meal in the present study. These differences are likely related to the reported left-shift of the concentration-time curve for faster aspart relative to IAsp, reflective of the faster onset and offset of action of faster aspart.13
While mealtime dosing of insulin is appropriate for many patients with diabetes, there are situations in which the increased flexibility of post-meal dosing may be advantageous. During social or other occasions, for example, the timing and/or carbohydrate content of a meal may be unpredictable. Furthermore, elderly or hospitalized patients, in whom lack of appetite and nausea are common, may also benefit from post-meal dosing, as might patients who have forgotten an injection, or are anxious about severe hypoglycaemia.18,19 Although post-meal dosing cannot be recommended, the results of the present study suggest that post-meal dosing of faster aspart may be considered in patients who need it.
This study employed once-daily degludec as the basal insulin. Degludec is associated with similar tolerability and overall glycaemic control compared to other long-acting insulin analogues, with the benefit of a reduced rate of overall symptomatic hypoglycaemic and severe hypoglycaemic events,20–22 which may have contributed towards the lower rates of hypoglycaemia observed here compared with onset 1.14 In addition, in the present study, an improvement in glycaemic control (reduction in HbA1c of ~0.6% in each arm) was achieved with weekly titration of degludec (pre-breakfast target 4.0–5.0 mmol/L) over the 8-week run-in period.
Strengths of onset 8 include the large patient cohort; the high percentage of participants completing the study; the randomized, double-blind design for the mealtime groups; and the individual optimization of basal insulin dose during the run-in period to allow a clearer comparison of the study arms. Furthermore, this is the first study to evaluate the combination of degludec, a basal insulin proven to confer fewer hypoglycaemic events, with faster aspart, and the use of faster aspart post-meal. The meal-test protocol was a potential limitation of the study, as the standardized meal composition may not accurately reflect what patients habitually consume. Moreover, all participants received an insulin dose of 0.1 U/kg for the meal test without any adjustment for individual insulin:carbohydrate ratios. The meal-test insulin dose was therefore only an approximation of the participant’s normal dose. Nevertheless, the reported SMBG 1-h PPG increment findings corroborated the meal-test results. Continuous glucose monitoring was not performed; however, its use in prospective studies could help to confirm hypoglycaemic events and evaluate the impact of treatment on glucose variability and time in range.
Conclusions
Together with the findings of onset 1, this study confirms that mealtime and post-meal faster aspart provide effective HbA1c control in people with T1D, as both met the criteria for non-inferiority compared with IAsp. Mealtime faster aspart further provides modestly improved control over early (30-min and 1-h) PPG excursions compared with mealtime IAsp. The overall safety profiles for faster aspart and IAsp are similar and as expected for the IAsp formulation. Administration of faster aspart after a meal, although less favourable compared with mealtime administration, may be considered for some people with T1D under certain circumstances.
Supplementary Material
Acknowledgments
We are grateful to the patients for their participation in the study. The onset 8 trial, including study design, data collection, analysis and interpretation, was funded by Novo Nordisk A/S. Medical writing and editorial assistance were provided by Steven Barberini PhD and Erin Slobodian of Watermeadow Medical, an Ashfield company, part of UDG Healthcare PLC, funded by Novo Nordisk A/S.
Footnotes
Conflict of interests
JBB has received contracted consulting fees, paid to his institution, and travel support from Adocia, AstraZeneca, Dexcom, Elcelyx Therapeutics, Eli Lilly, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Sanofi, Senseonics, and vTv Therapeutics and grant support from AstraZeneca, Boehringer Ingelheim, Johnson & Johnson, Lexicon, Novo Nordisk, Sanofi, Theracos and vTv Therapeutics. He is a consultant to Neurimmune AG. He holds stock options in Mellitus Health, PhaseBio and Stability Health. He is supported by a grant from the National Institutes of Health (UL1TR002489).
TK has received research funding (to his department) from Kowa Pharmaceutical, Mitsubishi Tanabe, MSD, Nippon Boehringer Ingelheim, Novo Nordisk, Ono Pharmaceutical, and Takeda; lecture fees from Astellas Pharma, AstraZeneca, Eli Lilly, Kissei Pharmaceutical, Kowa Pharmaceutical, Mitsubishi Tanabe, MSD, Nippon Boehringer Ingelheim, Novo Nordisk, Ono Pharmaceutical, Sumitomo Dainippon, and Takeda; fees for writing booklets from Eli Lilly; grants and endowments from Astellas Pharma, Daiichi Sankyo, Kissei Pharmaceutical, Kyowa Hakko Kirin, Mitsubishi Tanabe, Novartis, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku, Sumitomo Dainippon, Taisho Toyama Pharmaceutical, and Takeda; funds for contracted research from Daiichi Sankyo, Sanwa
Kagaku, and Takeda; and funds for collaborative research from Daiichi Sankyo and Novartis.
OM has participated in advisory boards for Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Jansen and Jansen, Novartis, Astra Zeneca; received grants paid to her institution for work as a study physician from AstraZeneca and Bristol-Myers Squibb, and research grant support through Hadassah Hebrew University Hospital from Novo Nordisk; and participated in speakers’ bureaus for AstraZeneca and Bristol-Myers Squibb, Novo Nordisk, Eli Lilly, Sanofi, Novartis, Merck Sharp & Dohme, and Boehringer Ingelheim.
MK has received research support from Nippon Boehringer Ingelheim Co. Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd., Msd K.K., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co. Ltd., Teijin Pharma Limited, Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Daiichi Sankyo Company, Limited, Fujifilm Pharma Co., Ltd., Eli Lilly Japan K., Taisho Toyama Pharmaceutical Co., Ltd., Novartis Pharma K.K., Kowa Pharmaceutical Co. Ltd; and participated in speakers’ bureaus for Nippon Boehringer Ingelheim Co. Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd.,Msd K.K., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Teijin Pharma Limited, Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Terumo Corporation, Daiichi Sankyo Company, Limited, Fujifilm Pharma Co., Ltd., Eli Lilly Japan K., Taisho Toyama Pharmaceutical Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin Ltd., Medtronic Japan Co., Ltd., Maruho Co. Ltd., Kowa Pharmaceutical Co. Ltd.
AC has participated in advisory boards for Sanofi, Insulet; acted as a consultant for Merck; and received research support from Medtronic and Novo Nordisk.
BL and KB are employees and stock/shareholders of Novo Nordisk A/S.
HH is an employee of Novo Nordisk Pharma Ltd.
LR has participated in advisory panels for Novo Nordisk; and acted as a consultant for and is a member of the Association of Statutory Health Insurance Physicians.
Clinical trial registry: NCT02500706, ClinicalTrials.gov
Data accessibility
The subject level analysis data sets for the research presented in the publication are available from the corresponding author on reasonable request.
Prior presentation
Parts of this paper have been accepted in poster form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018. Parts of this paper have also been submitted to 54th Annual Meeting of the European Association for the Study of Diabetes, 1–5 October 2018, Berlin, Germany; and the 21st Anniversary Diabetes Canada/CSEM Professional Conference, 10–13 October 2018, Halifax, NS, Canada.
References
- 1.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23):1676–1685. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S55–S64. [DOI] [PubMed] [Google Scholar]
- 5.Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). European heart journal. 2013;34(39):3035–3087. [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation Guideline Development Group. Guideline for management of postmeal glucose in diabetes. Diabetes research and clinical practice. 2014;103(2):256–268. [DOI] [PubMed] [Google Scholar]
- 7.Maiorino MI, Petrizzo M, Capuano A, Giugliano D, Esposito K. The development of new basal insulins: is there any clinical advantage with their use in type 2 diabetes? Expert Opin Biol Ther. 2014;14(6):799–808. [DOI] [PubMed] [Google Scholar]
- 8.Andersen G, Alluis B, Meiffren G, et al. Ultra-rapid BioChaperone insulin Lispro (BC-LIS): linear dose-response and faster absorption than insulin Lispro (LIS). Diabetologia. 2015;58 (Suppl 1):S449 (Abstract #931). [Google Scholar]
- 9.Boss AH, Petrucci R, Lorber D. Coverage of prandial insulin requirements by means of an ultra-rapid-acting inhaled insulin. J Diabetes Sci Technol. 2012;6(4):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazda C, Leohr J, Liu R, et al. A novel formulation of insulin lispro containing citrate and treprostinil shows faster absorption and improved postprandial glucose excursions vs Humalog in patients with T1DM. Diabetes. 2017;66((Suppl 1)):A229–A398. [Google Scholar]
- 11.Kapitza C, Leohr J, Liu R, et al. A novel formulation of insulin lispro shows significantly faster absorption and improvement in postprandial glucose excursions versus insulin lispro in patients with type 2 diabetes. Diabetologia. 2017;60((Suppl 1)):S1–S608. [Google Scholar]
- 12.Dodson M, Zhang C, Siesky A, et al. Exploration of the mechanism of accelerated absorption for a novel insulin lispro formulation. Diabetes. 2017;66((Suppl 1)):A229–A398. [Google Scholar]
- 13.Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clinical pharmacokinetics. 2017;56(5):551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell-Jones D, Bode BW, De Block C, et al. Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (onset 1). Diabetes Care. 2017;40(7):943–950. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu C, Bode BW, Franek E, et al. Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): a 52-week, randomized, treat-to-target, phase III trial. Diabetes Obes Metab. 2018;20(5):1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowering K, Case C, Harvey J, et al. Faster aspart versus insulin aspart as part of a basal-bolus regimen in inadequately controlled type 2 diabetes: the onset 2 trial. Diabetes Care. 2017;40(7):951–957. [DOI] [PubMed] [Google Scholar]
- 17.Rodbard HW, Tripathy D, Vidrio Velazquez M, Demissie M, Tamer SC, Piletic M. Adding fast-acting insulin aspart to basal insulin significantly improved glycaemic control in patients with type 2 diabetes: a randomized, 18-week, open-label, phase 3 trial (onset 3). Diabetes Obes Metab. 2017;19(10):1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cengiz E Undeniable need for ultrafast-acting insulin: the pediatric perspective. J Diabetes Sci Technol. 2012;6(4):797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ligthelm RJ, Kaiser M, Vora J, Yale JF. Insulin use in elderly adults: risk of hypoglycemia and strategies for care. J Am Geriatr Soc. 2012;60(8):1564–1570. [DOI] [PubMed] [Google Scholar]
- 20.Dzygalo K, Golicki D, Kowalska A, Szypowska A. The beneficial effect of insulin degludec on nocturnal hypoglycaemia and insulin dose in type 1 diabetic patients: a systematic review and meta-analysis of randomised trials. Acta Diabetol. 2015;52(2):231–238. [DOI] [PubMed] [Google Scholar]
- 21.Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: the SWITCH 1 randomized clinical trial. Jama. 2017;318(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377(8):723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.