Figure 10.
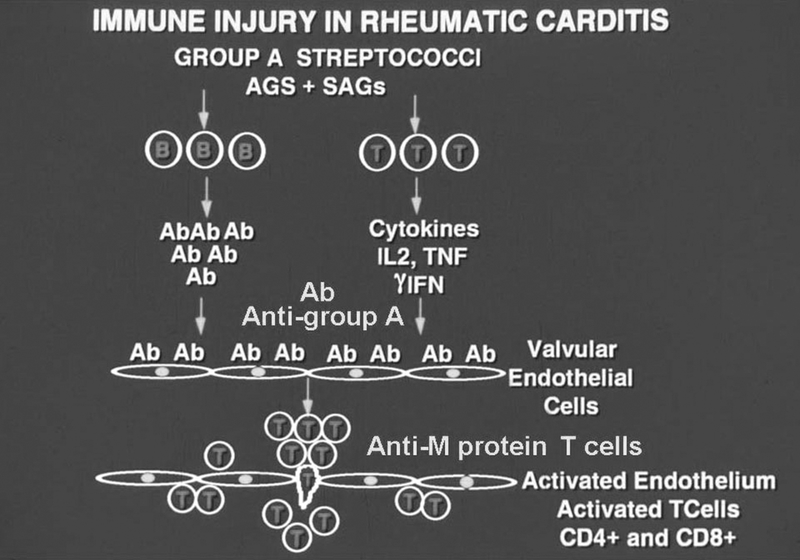
Diagram representing the proposed immunopathogenesis of post streptococcal rheumatic heart disease. Initially, B and T cells are activated by specific streptococcal antigens and superantigens leading to strong immune responses against streptococcal and host antigens. The development of pathogenic clones of B and T lymphocytes are important in development of the disease. Initially antibodies develop against the group A carbohydrate, which are cross-reactive with the valve surface and glycoproteins such as laminin and bind to the valve surface endothelium (endocardium) leading to inflammation and upregulation of cell adhesion molecules such as VCAM-1 on activated surface endothelium of the valve. M protein-reactive T cells enter the valve through the surface endothelium by binding to cell adhesion molecules such as VCAM-1 and extravasate into the valve (48). The formation of scar tissue in the valve followed by neovascularization allows for the disease to continue in the valve. The specificity of the T cells in blood (25)and T cells entering the valve has been shown to be M protein (21, 100, 144, 172). T cell subsets include Th1 (gamma IFN) (147) in the pathogenesis of proinflammatory responses and the development of the scarred and fibrotic valve. IL17A has also been associated with rheumatic heart disease in humans and animal models suggesting that Th17 cells are involved in disease(104, 149).
