Figure 6.
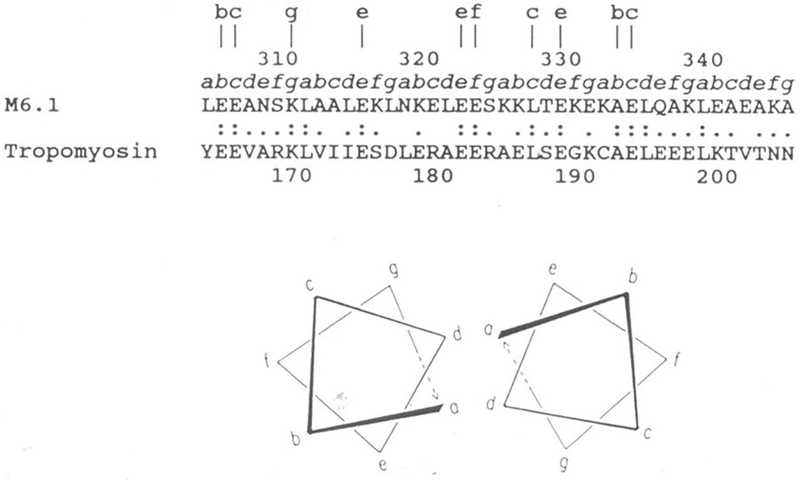
Sequence alignment of streptococcal M6 protein and human cardiac tropomyosin in a region exhibiting significant homology. Lower case letters a to g directly above the sequence designate the position of these amino acids within the seven-residue periodicity in both segments. Lower case letters at the top of the figure designate identities at external locations in the heptad repeat. Double dots indicate identities and single dots indicate conservative substitutions. Within this segment of the streptococcal M6 molecule, 31 percent homology is observed with tropomyosin. Since both molecules are alpha-helical coiled-coil proteins, they contain the seven-residue repeat pattern where positions a and d are usually hydrophobic. Similar homologies are seen between M proteins and myosin heavy chains and any of the three laminin chains. Reproduced from (40) with permission from the Journal of Immunology.
