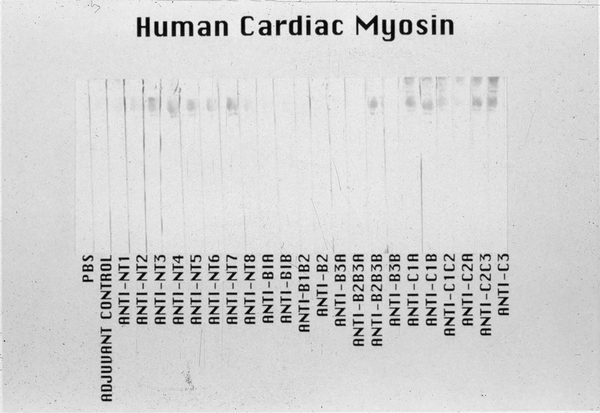
Figure 8.
Reactivity of the anti-streptococcal M5 peptide sera in the Western immunoblot of human cardiac myosin. The 200kD protein band of the purified human cardiac myosin, shown in the stained(S) portion of the Western blot, reacted most strongly with anti-peptide sera from mice immunized with M5 peptides NT3–7, B2B3B, and C1A, C1B, C2C3 and C3. Sera were tested at a 1:1000 dilution. A control anti-myosin mAb CCM-52 (a gift from Dr. William Clark, Cardiovascular Research Institute, Michael Reese Hospital and Medical Center, Chicago, IL) reacted with the 200kD band present in our purified preparation of human cardiac myosin heavy chain. Purification of the human cardiac myosin heavy chain to homogeneity has been previously described by Dell et al (209). The Western blot confirms the data seen in the ELISA with human cardiac myosin. Reproduced from (32) with permission from ASM Press.