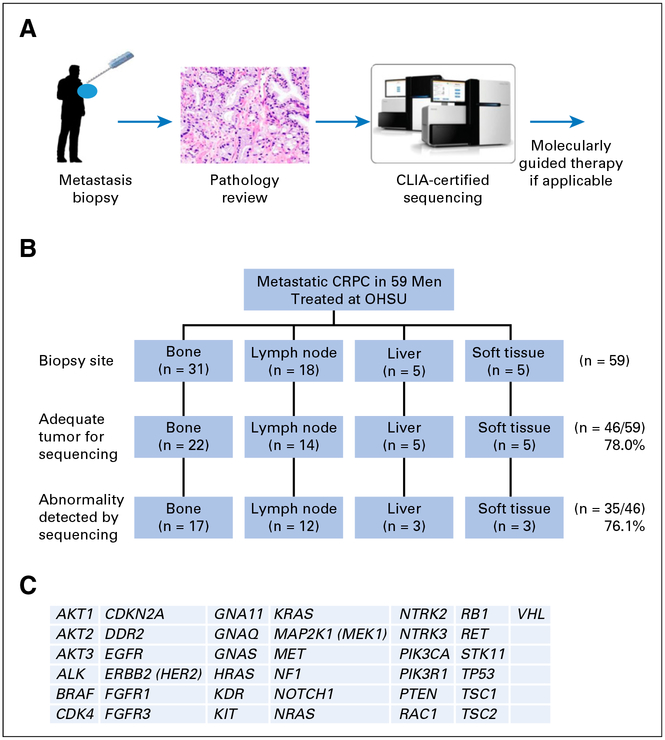
Fig 1.
Overview of the biopsy and clinical testing workflow. (A) Schema of biopsy and testing. (B) Biopsy sites of the samples used for Clinical Laboratory Improvement Amendments (CLIA)–approved sequencing and PTEN immunohistochemistry testing. (C) List of 37 genes tested for mutation in the CLIA-certified GeneTrails Solid Tumor Genotyping Panel. CRPC, castration-resistant prostate cancer; OHSU, Oregon Health & Science University.