Scheme 3.
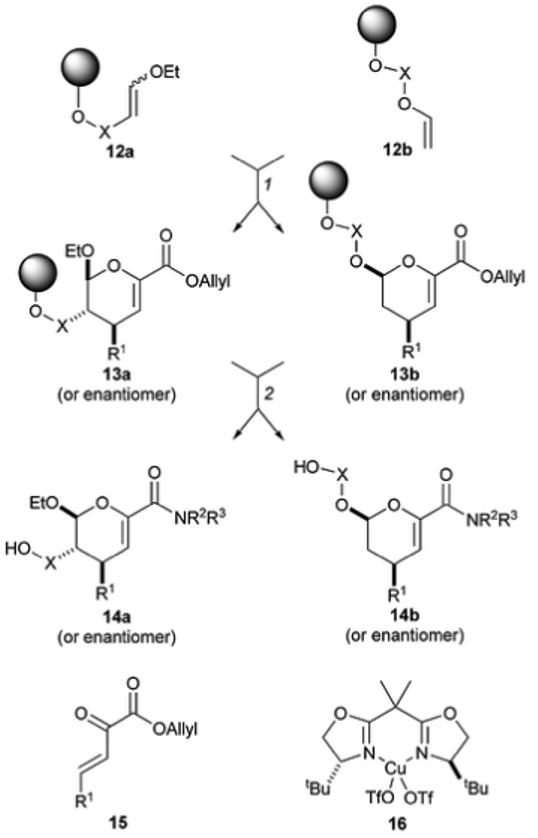
Schreiber’s synthesis of dihydropyrans with high substitutional and stereochemical diversity. Reagents and conditions: (1) 15 or 20 mol% 16 (or enantiomer), THF, rt; (2) (i) Pd(PPh3)4, thiosalicylic acid, THF, rt; (ii) R2R3NH, PyBOP, iPr2NEt, CH2Cl2-DMF, rt; (iii) HF·pyridine, THF, rt, then Me3SiOMe.
