Fig. 3.
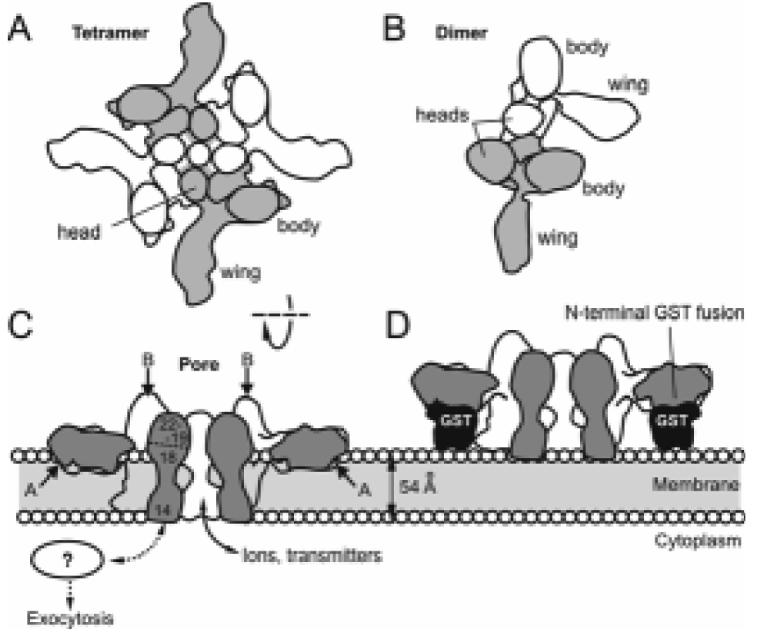
Dimers and tetramers of α-LTX and the mechanism of membrane pore formation. (A, B) Top views of the two major multimeric species of α-LTX. The monomers are coloured alternately. Both dimers and tetramers are able to bind receptors but only the tetramer forms integral membrane pores. (C) Cut-open side view of the α-LTX tetramer inserted into a lipid bilayer. The pore in the centre of the tetramer is permeable to ions and cytosolic neurotransmitters. Approximate positions of trypsin cleavage sites A and B (see text) are indicated with arrows. The locations of ARs 14-22 are shown as numbers in the cross-section of one monomer. (D) A predicted steric hindrance mechanism which may prevent membrane pore formation by N-terminal fusion constructs of α-LTX. Sections through glutathione-S-transferase (GST) are shown to scale.
