Figure 4.
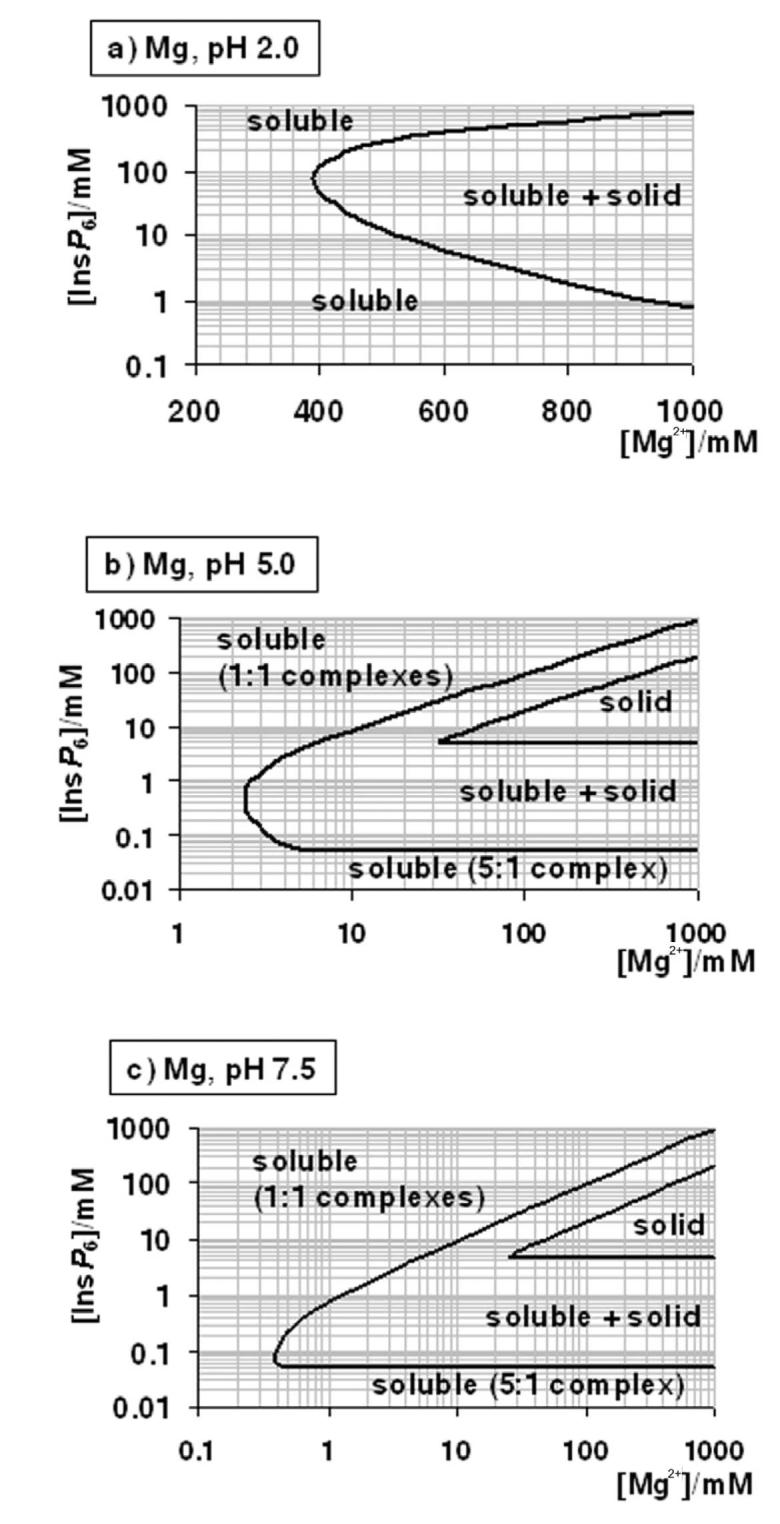
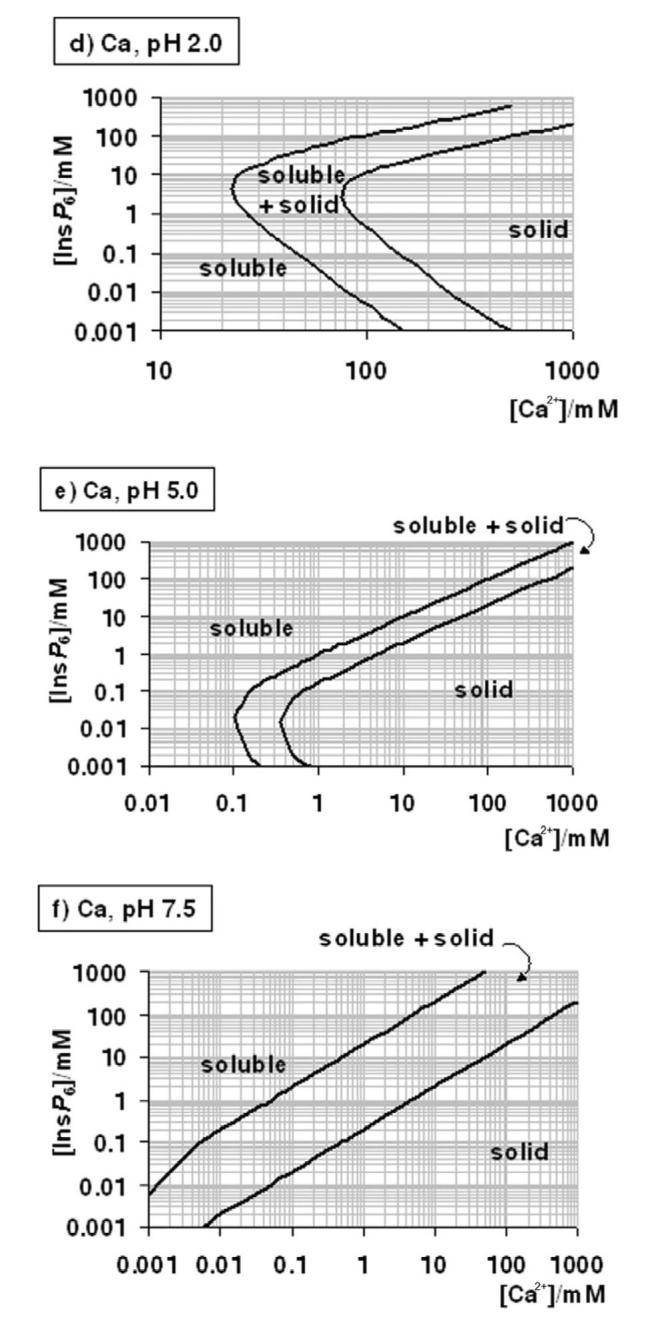
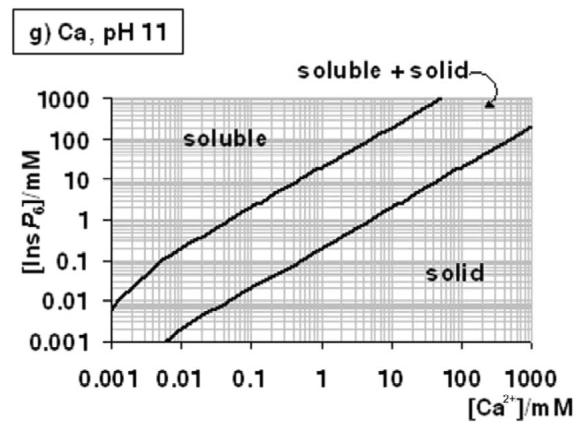
A “user's guide” to InsP6 in the presence of calcium and magnesium. The solubility behaviour of InsP6, as predicted by the equilibrium equations reported in this work and in [25], in the presence of Mg2+ (a-c) or Ca (d-g), is plotted for different pH values; plots for the Mg2+ system at basic pH values (above pH 7.5) are identical to that shown for pH 7.5. The “frontier lines” drawn correspond to conditions in which either 1% or 99% of InsP6 present is predicted to exist as a solid. Predictions were obtained by means of the HySS software, and are valid for I = 0.15 M NaClO4 and 37.0° C. In addition, the validity of the predictions may be limited by the potential precipitation, at high InsP6 concentrations, of salts containing Na+, Na+/Ca2+ or Na+/Mg2+, which have not been studied.
