Abstract
Cardiac diseases such as myocardial infarction and heart failure are among the leading causes of death in western societies. Therapeutic angiogenesis has been suggested as a concept to combat these diseases. The biology of angiogenic factors expressed in the heart such as vascular endothelial growth factor (VEGF) is well studied, whereas data on anti-angiogenic mediators in the heart are scarce. Here we study the expression of the anti-angiogenic factor pigment epithelium-derived factor (PEDF) in the human heart and in human cardiac cells. PEDF expression could be detected in human cardiac tissue on the protein and mRNA levels. PEDF mRNA levels were significantly lower in explanted human ischemic hearts as compared to healthy hearts. Our in vitro experiments showed that human adult cardiac myocytes and fibroblasts constitutively secrete PEDF. In addition to anoxic conditions, cobalt chloride, 2,2′dipyridyl and dimethoxally glycine, which stabilize hypoxia inducible factor-α decreased PEDF expression. Furthermore we show that PEDF inhibits VEGF-induced sprouting. We have identified PEDF in healthy and ischemic human hearts and we show that PEDF expression is down-regulated by low oxygen levels. Therefore, we suggest a role for PEDF in the regulation of angiogenesis in the heart and propose PEDF as a possible therapeutic target in heart disease.
Keywords: PEDF, anoxia, heart, angiogenesis
Introduction
Cardiac diseases such as myocardial infarction and heart failure are among the leading causes of death in western societies. One attractive and promising approach to combat excessive damage of the heart muscle after injury is to therapeutically modulate angiogenesis, the growth of new blood vessels. Accumulating evidence suggests a role for the heart as a source of growth factors and cytokines involved in the regulation of angiogenesis [1]. The biology of angiogenic factors expressed in the heart such as vascular endothelial growth factor (VEGF), various fibroblast growth factors (FGFs), hepatocyte growth factor (HGF) or angiopoietin-1 (Ang-1) has been extensively investigated. In contrast, studies on a possible involvement of anti-angiogenic mediators in the pathophysiology of cardiovascular disease are scarce [2].
Here we studied a possible role of the anti-angiogenic protein pigment epithelium-derived factor (PEDF) in the human heart. PEDF, a 50-kD non-inhibitory member of the serpin family, is widely expressed in various tissues throughout the body such as the skeletal muscle, adipose tissues, liver and bone and is also found in the plasma [3].
PEDF is a multifunctional protein with neurotrophic, neuroprotective, antioxidant, anti-inflammatory and also anti-angiogenic properties. It suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and proliferation of endothelial cells [4, 5]. Recently it has been shown that PEDF induces apoptosis in endothelial cells by activating FAS–FAS ligand death pathway and p38 MAP kinase dependent cleavage of caspase 3, 8 and 9 [3]. In addition, PEDF also inhibits angiogenesis by directly interfering with VEGF signalling via inducing cleavage of VEGF receptor 1 [6]. Interestingly the anti-angiogenic activity of PEDF is higher than that of other anti-angiogenic factors such as thrombospondin, angiostatin or endostatin [5].
In contrast to ocular pathologies and malignancies, information on a possible involvement of PEDF in cardiovascular pathologies is limited. PEDF inhibits occlusive thrombus formation in the carotid artery in a rat model and prevents neointimal formation in a rat carotid artery balloon injury model [7]. These findings suggest a possible protective role for PEDF in cardiovascular disease. In addition PEDF has been localized recently in atherosclerotic plaques [8]. In this study, the authors speculate that PEDF may affect the balance between angiogenesis and anti-angiogenesis during the development of the atherosclerotic lesion.
Here we investigate if PEDF is expressed in human cardiac tissue and regulated by anoxia in human cardiac cells. Furthermore we studied, additionally to the anti-angiogenic effect of PEDF, the expression pattern of PEDF in human healthy hearts and in hearts from heart failure patients suffering from ischemic and dilatative cardiomyopathy.
Material and methods
Preparation of human tissue
Human heart tissue was obtained from the left ventricle of explanted hearts from donors suffering from ischemic or dilatative cardiomyopathy and undergoing heart transplantation and from the left ventricle of healthy hearts unsuitable for transplantation. Tissue was stored at −80°C for RNA and protein isolation or embedded in paraffin for immunohistochemical staining. All human material was obtained and processed according to the recommendations of the hospital’s Ethics Committee including informed consent.
Western blotting
For Western Blotting human heart tissue was homogenized using a ball mill (Retsch, Haan, Germany) and incubated for 1 hr with urea (8 M; Sigma, St. Louis, MO, USA) at 4°C. For equal protein loading total protein concentration was determined using Bradford reagent (BioRad, Hercules, CA, USA). We used an anti-human goat PEDF antibody (0.2 μg/ml; R&D Systems, Minneapolis, MN, USA) and an anti-human rabbit hypoxia inducible factor-1α (HIF-1α) antibody (1/500 dilution, Cell Signaling, Beverly, MA, USA) as primary and a donkey anti-goat IgG-HRP (0.2 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a donkey anti-rabbit IgG-HRP (1/10000 dilution, GE Health Care, Chalfont St. Giles, UK) as secondary antibodies, respectively. Proteins were detected with ECL system (GE Health Care).
Real-time PCR
Frozen human cardiac tissue was homogenized using a ball mill (Retsch). High Pure RNA Tissue Kit was used for mRNA isolation from tissue and High Pure RNA Isolation Kit (both Roche, Basel, Switzerland) for cell culture. Real-time PCR was performed with LightCycler-RNA Master SYBR Green I (Roche) according to the manufacturer’s instructions. Primers were designed using Primer3 Software (http://frodo.wi.mit.edu/): GAPDH-forward primer (human): 5′-acagtccatgccatcactgcc-3′, GAPDH-reverse primer (human): 5′-gcctgcttcaccaccttcttg -3′, PEDF-forward primer (human): 5′- ctgcagggacttggtgactt-3′, PEDF-reverse primer (human): 5′- gtcggaccctaaggctgttt -3′. The amplification conditions consisted of an initial incubation at 61°C for 20 min., followed by incubation at 95°C for 30 sec., 50 cycles of 95°C for 1 sec., the respective annealing temperature (67°C for human PEDF, 65°C for human GAPDH) for 10 sec. and 72°C for 10 sec., a melting step from 45°C to 95°C increasing 0.1°C per second and a final cooling to 40°C. Data were analysed using LightCycler Software Version 3.5 (Roche, Switzerland). For quantification we employed the comparative CT method. Values were normalized to GAPDH levels. Agarose gel (3%) electrophoresis was performed to visualize PCR products.
Immunohistochemistry
Paraffin embedded human heart tissue was stained using the MultiLink® horse radish peroxidase system (BioGenex, San Ramon, CA, USA) according to the manufacturer’s instructions. We used an anti-human PEDF goat antibody (15 μg/ml; R&D Systems) for PEDF staining, an anti-human cardiac troponin I rabbit monoclonal antibody (1/250 dilution; Abcam, Cambridge, UK) for cardiac muscle staining and an anti-human smooth muscle α-actin mouse monoclonal antibody (ready to use, BioGenex). As a control we stained without using the first antibodies. For PEDF antigen retrieval samples were boiled for 10 min. at 1 bar in a high pH target retrieval solution (pH = 10; Dako, Glostrup, Denmark). Slides were counterstained with haematoxylin (Merck, Darmstadt, Germany).
Isolation and cultivation of human cells
Human adult cardiac myocytes (HACM), human adult cardiac fibroblasts (HACF) and human umbilical vein endothelial cells (HUVEC) were isolated, cultivated and characterized as previously described [9, 10].
Twenty-four hours prior to experiments confluent cells were starved with M199 containing 0.1% bovine serum albumin (Sigma). For anoxia experiments cells were cultivated in an anaerocult® IS incubation bag (Merck). Additionally cells were stimulated with cobalt chloride (CoCl2), 2,2′dipyridyl (DP, both Sigma) or dimethoxally glycine (DMOG, Cayman Chemical, Ann Arbor, MI, USA) at the given concentrations for 6, 8 or 48 hrs. We treated these cardiac cells also with 100 ng/ml recombinant human (rh) PEDF (Millipore, Billerica, MA, USA) for 48 hrs.
Protein determination
PEDF and VEGF protein in the supernatants of the cell culture was determined using a specific ELISA for PEDF (MDBioproduct, Middletown, MD, USA) and VEGF (R&D Systems), respectively, according to the manufacturer’s instructions.
Transcription factor activation
For nuclear extraction, the NE-PER® nuclear extraction reagents and Halt™ protease inhibitor cocktail (both Pierce, Rockford, IL, USA) were used. Cells were manually scratched and isolation of the nuclear fraction was performed according to the manufacturer’s instructions. Nuclear extracts were immediately snap frozen and stored at −80°C until used. Total protein content of the nuclear extract was determined using Bradford Reagent. For determination of transcription factor activity for HIF-1α the TransAM™ ELISA system (Active Motif, Rixensart, Belgium) was used according to the manufacturer’s instruction.
Spheroid sprouting assay
The in vitro angiogenesis assay using collagen gel-embedded HUVEC spheroids was performed as described by Korff et al. [11]. The collagen gel-embedded spheroids were stimulated with 50 ng/ml rh VEGF (R&D Systems), 500 ng/ml rh PEDF or both for 24 hrs. Spheroid sprouting was analysed measuring the total area of spheroids and mean distance of sprouts from the focal point (S.CO LifeScience GmbH, Garching, Germany).
Statistical analysis
Experiments were repeated at least three times, one representative experiment is shown. Data were compared statistically by t-test (independent variables). Values of P < 0.05 were considered significant.
Results and discussion
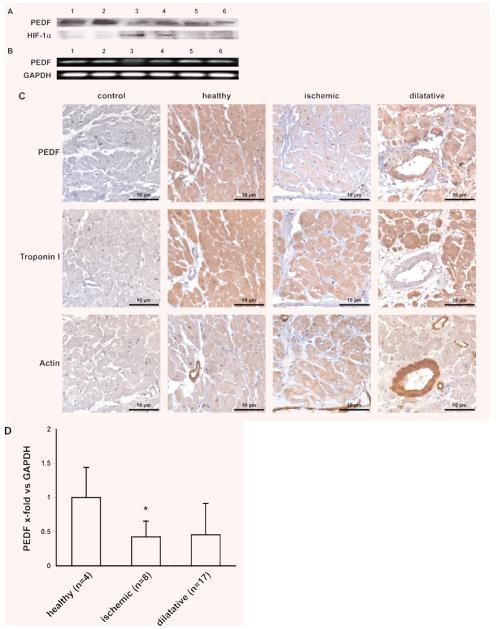
We present here evidence that the potent anti-angiogenic protein PEDF is expressed in human hearts. As revealed by Western blotting and PCR, we show that PEDF protein and mRNA is present in the human heart (Fig. 1A and B). The highest levels of PEDF protein were found in healthy hearts whereas these levels were reduced in heart tissue from patients suffering from ischemic or dilatative cardiomyopathy (Fig. 1A). PEDF levels as measured by a specific ELISA in these samples were highest in the healthy hearts (2.6 ng/μg tissue), lowest in the ischemic hearts (1.6 ng/μg tissue) and intermediate in dilated hearts (1.9 ng/μg tissue). In contrast, levels of HIF-1α protein, which were studied to evaluate the ischemic state of the tissue, were highest in ischemic hearts (Fig. 1A). mRNA levels for PEDF in ischemic hearts were lower as compared to mRNA levels in healthy hearts and in dilated hearts (Fig. 1B). The results for PEDF protein were confirmed by immunohistochemical staining using paraffin embedded heart tissue (Fig. 1C). Strongest staining for PEDF was observed in sections obtained from healthy hearts, whereas the sections obtained from ischemic heart exhibited the weakest staining. Intermediate staining was seen in heart tissue from patients suffering from dilatative cardiomyopathy. Stains of serial sections showed that myocardial cells positive for PEDF protein also stained positive for cardiac troponin I. Using these sections we detected PEDF also in human vascular smooth muscle cells, which stained positive for smooth muscle α-actin and which had been identified as a source for PEDF recently [8]. In order to quantitatively analyse these findings in more depth we performed quantitative real-time PCR to compared PEDF mRNA levels in the left ventricle of explanted hearts from heart failure patients suffering from ischemic (n = 8) or dilatative (n = 17) cardiomyopathy to levels of PEDF mRNA in healthy human hearts (n = 4) unsuitable for heart transplantation. We could show that PEDF mRNA levels were significantly reduced in patients suffering from ischemic cardiomyopathy as compared to healthy hearts. These mRNA levels were also reduced in patients suffering from dilatative cardiomyopathy. However, this latter decrease did not reach significance (Fig. 1D).
Fig. 1.
PEDF expression in human cardiac tissue. PEDF and HIF-1α determination by Western blot of homogenized cardiac tissue isolated from two healthy hearts (lanes 1 and 2) and four explanted hearts from patients suffering from ischemic (lanes 3 and 4) and dilatative (lanes 5 and 6) cardiomyopathy (A). PEDF mRNA expression in human cardiac tissue isolated from the same hearts used for Western blot in (A), e.g. two healthy hearts (lanes 1 and 2) and four explanted hearts from patients suffering from ischemic (lanes 3 and 4) and dilatative (lanes 5 and 6) cardiomyopathy; GAPDH served as a loading control (B). Immunohistochemical staining of PEDF, troponin I and actin in paraffin embedded heart tissue from a healthy heart and explanted hearts from patients suffering from ischemic and dilatative cardiomyopathy, respectively (C). mRNA was isolated from the left ventricle of healthy human hearts (n = 4) and from hearts of patients suffering from ischemic (n = 8; *P = 0.014) or dilatative cardiomyopathy (n = 17; n.s., P = 0.287); real-time PCR was performed employing specific primers for PEDF. Values represent mean values ± S.D. Values are given as x-fold of control and were normalized using GAPDH levels (D).
Based on our in vivo data we isolated and cultivated HACM and HACF and analysed PEDF expression in these cells. We could show that both cardiac cell types constitutively express PEDF. Under anoxic conditions PEDF expression was reduced down to 50% on average in these cells. These results were confirmed in myocytes and fibroblasts isolated from four different donors (Table 1). If another cell type, recently described to be present in cardiac tissue, namely interstitial Cajal-like cells, also expresses PEDF remains to be investigated [12]. A decrease in PEDF production due to low oxygen concentration has been recently described in other cell types like retinoblastoma cells and retinal glial cells, albeit in retinoblastoma cells only on the protein level and not on mRNA level [5, 13]. Due to low oxygen concentration the HIF is stabilized and transported to the nucleus [14]. To test if HIF-α was involved in the down-regulation of PEDF, we treated these cardiac cells with CoCl2, DP and DMOG, which are known to stabilize HIF-α by different mechanisms and therefore mimic anoxic conditions [15–17]. Similar to anoxia, CoCl2, DP and DMOG reduced PEDF expression dose dependently in cardiac myocytes and fibroblasts, suggesting that HIF-α might be involved in the decrease of PEDF (Fig. 2A and B). These data were confirmed at mRNA level (Fig. 2C). Furthermore we showed an increased accumulation of HIF-1α in the nuclear extract of cardiac myocytes and fibroblasts stimulated with CoCl2, DP and DMOG or cultivated under anoxic conditions (Fig. 2D). In addition we detected increased HIF-1α expression in the lysate of these cells (data not shown). There is convincing evidence that the expression of the proangiogenic factor VEGF is strongly increased by ischemia [14]. In agreement with published data we detected increased VEGF levels in human cardiac cells cultivated under anoxic conditions (Table 1), resulting in an increased VEGF/PEDF ratio [14]. The reciprocal regulation of PEDF and VEGF by anoxia has also been reported in the retina and in retinal cells [5, 13]. Furthermore Zhang et al. showed that PEDF down-regulated VEGF expression under normoxic and anoxic conditions in retinal cells [18]. Interestingly, when we incubated human cardiac myocytes and fibroblasts with 100 ng/ml PEDF no effect on VEGF expression was observed (HACM control: 3.750 ± 0.637, PEDF: 3.095 ± 0.636, anoxia: 17.734 ± 3.586, anoxia + PEDF: 20.176 ± 0.890; HACF: control: 2.987 ± 0.280, PEDF: 2.988 ± 0.120, anoxia: 9.686 ± 0.146, anoxia + PEDF: 9.936 ± 0.099; values represent mean values ± S.D. of three independent determinations and are given in ng/105 cells/48 hrs), suggesting that PEDF-induced regulation of VEGF could be cell type specific.
Table 1.
PEDF and VEGF production in human cardiac cells under normoxic and anoxic conditions
| HACM | PEDF | VEGF | ||
|---|---|---|---|---|
| Control | Anoxia | Control | Anoxia | |
| Donor 1 | 19.240 ± 0.413 | 9.511 ± 0.997* | 3.707 ± 0.939 | 7.193 ± 0.448* |
| Donor 2 | 18.056 ± 1.427 | 10.160 ± 1.131* | 3.215 ± 0.079 | 8.129 ± 0.716* |
| Donor 3 | 16.700 ± 1.124 | 8.350 ± 0.220* | 3.257 ± 0.252 | 10.969 ± 0.845* |
| Donor 4 | 15.468 ± 0.209 | 8.315 ± 0.243* | 3.824± 0.633 | 21.970 ± 0.367* |
| HACF | PEDF | VEGF | ||
|---|---|---|---|---|
| Control | Anoxia | Control | Anoxia | |
| Donor 1 | 10.235 ± 1.129 | 6.209 ± 0.217* | 2.876 ± 0.884 | 6.706 ± 0.812* |
| Donor 2 | 5.712 ± 0.252 | 2.655 ± 0.156* | 4.549 ± 0.338 | 12.560 ± 0.326* |
| Donor 3 | 7.806 ± 0.105 | 4.196 ± 0.123* | 3.181 ± 0.559 | 14.138 ± 1.051* |
| Donor 4 | 7.432 ± 0.856 | 3.783 ± 0.674* | 2.661 ± 0.619 | 7.772 ± 0.710* |
HACM and HACF isolated from explanted hearts from patients suffering from ischemic (donors 1and 2) and dilatative cardiomyopathy (donors 3 and 4) were cultivated under normoxic (control) and anoxic conditions for 48 hrs; PEDF and VEGF in the supernatant was determined by specific ELISA, values are given in ng/100,000 cells. Values represent mean values ± S.D. of three independent determinations.
P < 0.005.
Fig. 2.
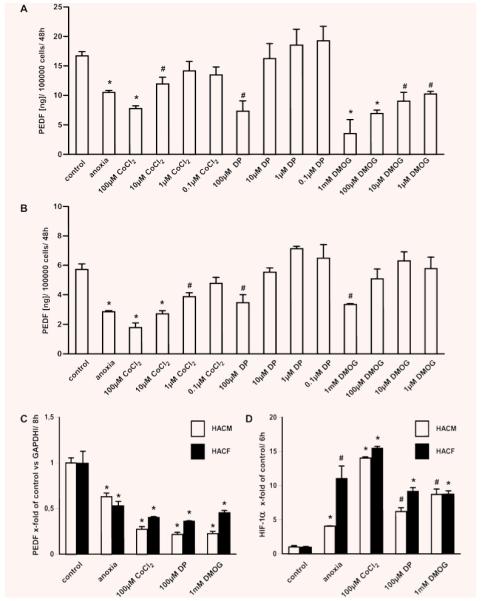
Regulation of PEDF in human cardiac cells. HACM (A) and HACF (B) were cultivated under normoxic (control) and anoxic conditions or stimulated with CoCl2 (0.1–100 μM), DP (0.1–100 μM) or DMOG (1 mM to 1 μM) for 48 hrs; PEDF protein in conditioned media was determined by specific ELISA. Values are given as ng/100,000 cells and represent mean values ± S.D. of three independent determinations (A, B). mRNA was isolated from HACM and HACF cultivated for 8 hrs under normoxic (control) or anoxic conditions or stimulated with 100 μM CoCl2, 100 μM DP or 1 mM DMOG. Real-time PCR for PEDF was performed employing specific primers. Values, given as x-fold of control, represent mean values ± S.D. of three independent determinations and were normalized to GAPDH levels (C). HACM and HACF were cultivated for 6 hrs under normoxic (control) or anoxic conditions or stimulated with 100 μM CoCl2, 100 μM DP or 1 mM DMOG; nuclear extract was isolated and HIF-1α activation was measured using a specific transcription factor ELISA. Values represent mean values ± S.D. of three independent determinations and are given as x-fold of control (D). *P < 0.005, #P < 0.05.
To evaluate the anti-angiogenic effect of PEDF [4, 5] we performed a spheroid sprouting assay. PEDF inhibited VEGF-induced sprouting of spheroids (Fig. 3A). The total area of spheroids stimulated with PEDF and VEGF and the mean distance of the sprouts from the focal point were significantly reduced compared to VEGF stimulated spheroids. PEDF alone did not show any effect (Fig. 3B and C). Our results are in line with the notion that PEDF directly affects VEGF-induced angiogenesis [19].
Fig. 3.
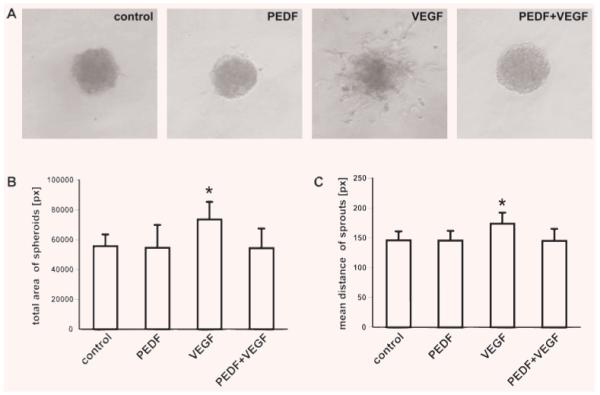
PEDF inhibits VEGF-induced sprouting. Spheroids were cultivated in the absence (control) or presence of PEDF, VEGF or both PEDF and VEGF for 24 hrs (A). Sprouting was analysed measuring the total area (pixel) of the spheroids (n = 8) (B) and the mean distance (pixel) of the sprouts from the focal point (C). Values represent mean values ± S.D. *P < 0.05.
Taken together we present here evidence that the potent anti-angiogenic protein PEDF is present in human hearts and that it is constitutively expressed by cardiac myocytes and fibroblasts. Furthermore, we showed that PEDF protein and mRNA levels are down-regulated by anoxia in these cells and that HIF-1α might modulate this effect. The notion that anoxia could be a key trigger of PEDF down-regulation in the heart is further supported by our observation that PEDF is significantly down-regulated in human ischemic hearts.
In contrast to angiogenic mediators such as VEGF and FGFs, whose presence and regulation in the heart have been extensively studied, information on anti-angiogenic factors in the heart is scarce [1]. Recently, expression of endostatin and angiostatin has been described in diabetic porcine myocardium [20]. Here we have identified yet another anti-angiogenic mediator, namely PEDF, in the heart. Given its regulated presence in the human heart and its potent anti-angiogenic effect, PEDF could be considered as a promising candidate for therapeutic modulation of angiogenesis. Using PEDF therapeutically to inhibit angiogenesis or induce vessel regression has already been suggested in diseases like cancer, retinopathy or arthritis [3]. On the other hand blocking PEDF efficiently in the heart – possibly in concert with simultaneous administration of angiogenic factors or stem cells – could result in increased revascularization, healing and regeneration of ischemic heart tissue with beneficial consequences for patients suffering from ischemic heart disease [21, 22]. However, as our study is limited to the expression and regulation of PEDF in the heart, further studies are warranted to more closely define its function and pathophysiological role in this organ.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF JRP ‘Angiogenesis in Disease’ S9409-B11), by the Ludwig Boltzmann Cluster for Cardiovascular Research and by the Association for the Promotion in Research in Arteriosclerosis, Thrombosis and Vascular Biology.
References
- 1.Tomanek RJ, Zheng W, Yue X. Growth factor activation in myocardial vascularization: therapeutic implications. Mol Cell Biochem. 2004;264:3–11. doi: 10.1023/b:mcbi.0000044369.88528.a3. [DOI] [PubMed] [Google Scholar]
- 2.Molin D, Post MJ. Therapeutic angiogenesis in the heart: protect and serve. Curr Opin Pharmacol. 2007;7:158–63. doi: 10.1016/j.coph.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003;4:628–36. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 4.Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43:821–9. [PubMed] [Google Scholar]
- 5.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Jiang WG, Grant MB, et al. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–13. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, Yamagishi S, Matsui T, et al. Pigment epithelium-derived factor inhibits neointimal hyperplasia after vascular injury by blocking NADPH oxidase-mediated reactive oxygen species generation. Am J Pathol. 2007;170:2159–70. doi: 10.2353/ajpath.2007.060838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baba H, Yonemitsu Y, Nakano T, et al. Cytoplasmic expression and extracellular deposition of an antiangiogenic factor, pigment epithelium-derived factor, in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2005;25:1938–44. doi: 10.1161/01.ATV.0000175759.78338.1e. [DOI] [PubMed] [Google Scholar]
- 9.Hohensinner PJ, Kaun C, Rychli K, et al. Monocyte chemoattractant protein (MCP-1) is expressed in human cardiac cells and is differentially regulated by inflammatory mediators and hypoxia. FEBS Lett. 2006;580:3532–8. doi: 10.1016/j.febslet.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Tal Cohen E, Hohensinner PJ, Kaun C, et al. Statins decrease TNF-alphainduced osteoprotegerin production by endothelial cells and smooth muscle cells in vitro. Biochem Pharmacol. 2007;73:77–83. doi: 10.1016/j.bcp.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112:3249–58. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 12.Popescu LM, Gherghiceanu M, Hinescu ME, et al. Insights into the interstitium of ventricular myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–58. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange J, Yafai Y, Reichenbach A, et al. Regulation of pigment epithelium-derived factor production and release by retinal glial (Muller) cells under hypoxia. Invest Ophthalmol Vis Sci. 2008;49:5161–7. doi: 10.1167/iovs.08-2201. [DOI] [PubMed] [Google Scholar]
- 14.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Y, Hilliard G, Ferguson T, et al. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem. 2003;278:15911–6. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
- 16.Natarajan R, Salloum FN, Fisher BJ, et al. Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am J Physiol Heart Circ Physiol. 2007;293:H1571–80. doi: 10.1152/ajpheart.00291.2007. [DOI] [PubMed] [Google Scholar]
- 17.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang SX, Wang JJ, Gao G, et al. Pigment epithelium-derived factor down-regulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 19.Ek ET, Dass CR, Choong PF. PEDF: a potential molecular therapeutic target with multiple anti-cancer activities. Trends Mol Med. 2006;12:497–502. doi: 10.1016/j.molmed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Boodhwani M, Sodha NR, Mieno S, et al. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I31–7. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesselmann C, Ma N, Bieback K, et al. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008;12:1795–810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tateishi K, Takehara N, Matsubara H, et al. Stemming heart failure with cardiacor reprogrammed-stem cells. J Cell Mol Med. 2008;12:2217–32. doi: 10.1111/j.1582-4934.2008.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]