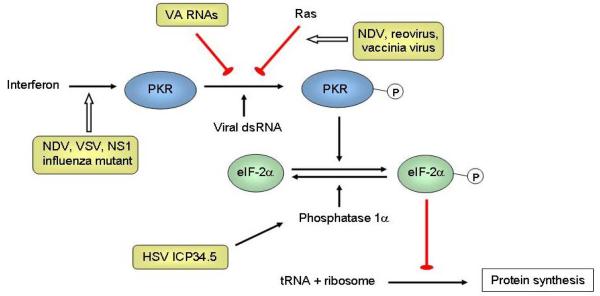
Figure 1.
Mechanisms of tumor selectivity of several oncolytic viruses. The interferon (IFN)/double-stranded RNA-activated protein kinase (PKR) pathway is a natural anti-viral defense system. IFNs produced by infected cells result in the upregulation of PKR. On binding to viral double-stranded RNA (dsRNA), PKR autophosphorylates, which in turn phosphorylates the α subunit of eIF-2. Phosphorylated eIF-2α sequesters eIF-2B, a guanine nucleotide exchange factor. Without eIF-2B, the GDP bound to eIF-2 cannot be exchanged for GTP. As a result eIF-2 is unable to bring the initiator transfer RNA (tRNA) to the 40S ribosomal subunit, and the synthesis of viral protein is inhibited. Inactivated IFN and activated Ras pathways are frequently found in cancer (the latter could inhibit PKR), and some naturally-found viruses can replicate selectively in cancer but not normal cells, including the Newcastle disease virus (NDV) [21], reovirus [22], vaccinia virus [23], and vesicular stomatitis virus (VSV) [24]. The herpes simplex virus (HSV) protein ICP34.5 interacts with cellular phosphatase 1α to dephosphorylate eIF-2α, leading to synthesis of proteins needed for virus replication. Deletion of gene that encodes for ICP34.5 (RL1) results in selective replication in tumors with a defective IFN/PKR pathway [25]. The influenza virus NS1-deleted mutant is also dependent on this defective pathway [26]. Adenoviruses normally produce virus-associated (VA) RNAs to inhibit PKR. As such, engineered VAI-deleted adenovirus (dl331) could replicate selectively in tumors with an activated Ras pathway [27]. Epstein-Barr virus (EBV) also expresses RNAs similar to VA RNAs and these can complement dl331, resulting in selectivity in EBV-associated tumors [28].