Table 1.
List of nonfluorescent chlorophyll catabolites (NCCs) from higher plants with a common general formula and varying modifications R1, R2 and R3.[a]
| Compound | R1 | R2 | R3 | Ref. | ||
|---|---|---|---|---|---|---|
| 1 | Hv-NCC-1 | OH | CH3 | CH(OH)–CH2OH | [3,12] |
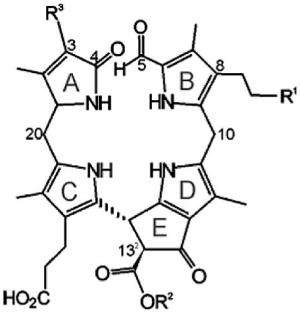
|
| 2 |
Cj-NCC-1/So-NCC-4/ Pc-NCC-2/Ms-NCC-2 |
OH | CH3 | CH=CH2 | [18-20,47] | |
| 3 | Cj-NCC-2/So-NCC-5 | H | CH3 | CH=CH2 | [18-20] | |
| 4 | Bn-NCC-1 | O-Mal | H | CH=CH2 | [13,14] | |
| 5 | Bn-NCC-2/At-NCC-1 | O-β-(6′-O-Mal)Glc | H | CH=CH2 | [14,23] | |
| 6 | Bn-NCC-3/At-NCC-2 | OH | H | CH=CH2 | [14,23] | |
| 7 | Bn-NCC-4/At-NCC-5 | H | H | CH=CH2 | [23] | |
| 8 | At-NCC-4 | O-β-Glc | CH3 | CH=CH2 | [23] | |
| 9 | So-NCC-1 | OH | H | CH(OH)–CH2OH | [18] | |
| 10 | So-NCC-2 | OH | CH3 | CH(OH)–CH2OH | [17,18] | |
| 11 | So-NCC-3 | OH | H | CH=CH2 | [18] | |
| 12 | Nr-NCC-1 | O-β-(6′-O-Mal)Glc | CH3 | CH=CH2 | [15] | |
| 13 |
Nr-NCC-2/Zm-NCC-2 Pc-NCC-1 |
O-β-Glc | CH3 | CH=CH2 | [15,16,47] | |
| 14 | Zm-NCC-1 | O-β-Glc | CH3 | CH(OH)–CH2OH | [16] |
Abbreviations: Mal = malonyl; Glc = glucopyranosyl.
