Table 1.
GRADE summary combining quality of evidence and summary of findings*
Question: What clinical signs best identify severe illness in young infants aged 0–59 days?
Settings: Primary healthcare settings in resource-poor settings
Diagnostic criteria: Clinical signs (clinical referral algorithms)
| Quality assessment |
Summary of findings |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | No of infants | Design | Limitations | Inconsistency | Indirectness | Imprecision | ORs† (Range) |
Quality (GRADE) |
Importance |
| Cyanosis§ | |||||||||
| 3 4 6 13 | 13 428 | Observational studies |
No serious limitations |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
1.5–25.8 |
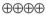 HIGH |
Critical |
| Change in level of activity‡ | |||||||||
| 3 6 11 13 | 15 759 | Observational studies |
No serious limitations |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
1.5–15.1 |
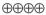 HIGH |
Critical |
| Fast breathing (respiratory rate ≥60 bpm) | |||||||||
| 3 4 6 13 | 13 428 | Observational studies |
No serious limitations |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
1.5–3.1 |
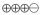 MODERATE |
Critical |
| Grunting | |||||||||
| 2 6 13 | 12 192 | Observational studies |
No serious limitations |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
1.5–2.9 |
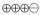 MODERATE |
Critical |
| History of convulsions | |||||||||
| 2 6 13 | 12 192 | Observational studies |
No serious limitations |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
1.5–15.4 |
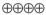 HIGH |
Critical |
| History of difficulty feeding | |||||||||
| 3 4 6 13 | 13 428 | Observational studies |
No serious limitations |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
1.5–10.0 |
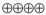 HIGH |
Critical |
| Severe chest indrawing | |||||||||
| 4 4 6 12 13 | 13 939 | Observational studies |
No serious limitations |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
1.5–8.9 |
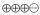 MODERATE |
Critical |
| Temperature (axillary) ≥37.5°C or <35.5°C | |||||||||
| 3 4 6 13 | 13 428 | Observational studies |
No serious limitations |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
1.5–9.2 |
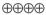 HIGH |
Critical |
Quality of evidence—the extent to which we can be confident that an estimate of effect or association is correct. The judgements are based on the: study design (randomised vs observational studies); likelihood of bias; consistency of the results across the studies; precision (wide or narrow CIs) of overall estimates and; directness of the evidence with respect to the populations, interventions and settings where the proposed intervention may be used
ORs of signs or symptoms calculated by multivariable analyses
History of reduced activity, showing no spontaneous movement, stiff limbs, limps becoming limp
Bluish or greyish discoloration of the tongue
- HIGH : Further research is very unlikely to change our confidence in the estimate of effect.
- MODERATE : Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
- LOW: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
- VERY LOW : We are very uncertain about the estimate.
