Fig. 5.
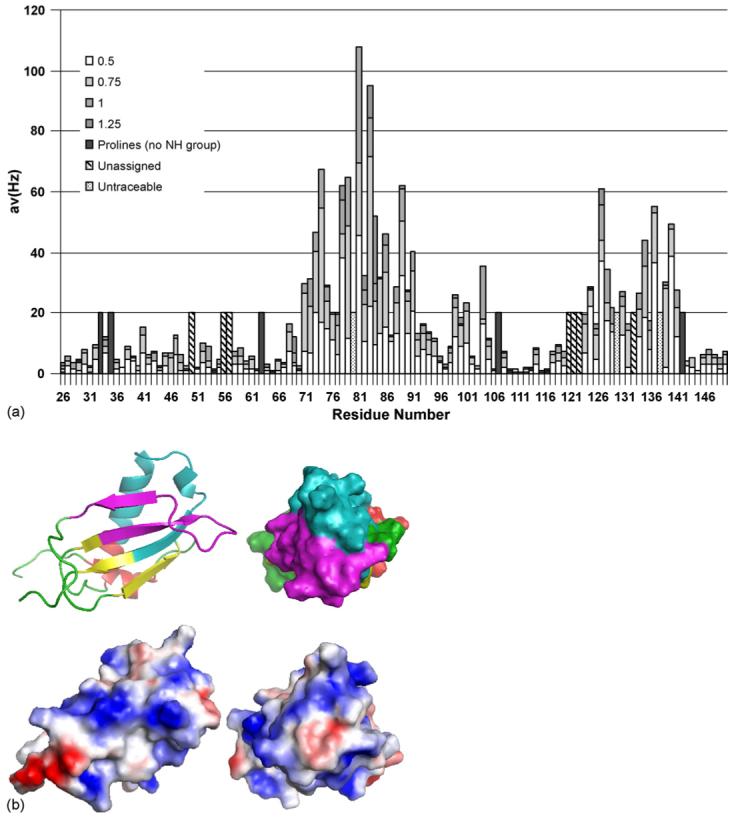
Analysis of the interaction of CHIPS with the N-terminus of C5aR. (a) Averaged chemical shift changes of the amide cross peaks of 15N CHIPS28-149 upon complex formation with human C5aR1-37 peptide, corresponding to the N-terminal extracellular region of the C5a receptor. The chemical shift changes were calculated using the formula , where the shift differences (Δδ) between free and complexed form are observed by two-dimensional 1H−15N HSQC spectra. (b) Mapping of CHIPS regions interacting with hC5aR1-37. Top panels: residues 71-97 (cyan) and 123-140 (magenta), shown by NMR chemical shift perturbation to be involved in interaction with hC5aR1-37, are mapped on to the structure of rCHIPS31-121 (Haas et al., 2005). The rest of the rCHIPS31-121 structure is displayed in yellow (β-strands), red (α-helices), and green (loops). Bottom panels: electrostatic surface view of the representations above. Blue and red represent positively and negatively charged surface.
