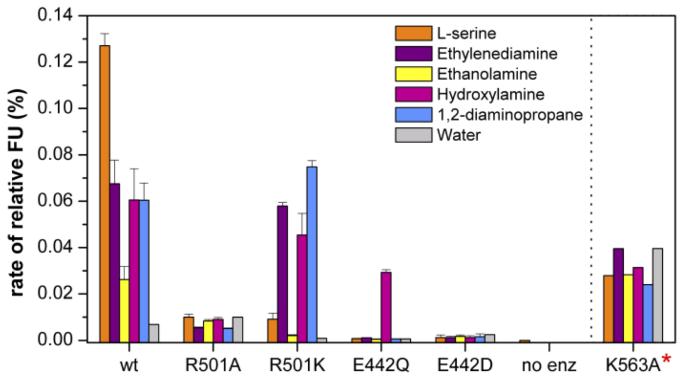
Figure 4.
Mutation of residues predicted to be important in recognition and catalysis of the second step of the transformation mediated by AcsD. The specific activity is shown as fluorescence units (FU) on the Y-axis. The data for the K563A mutant overestimate the specific activity, because the protein was not fully purified due to low levels of expression. The R501K mutant shows a reversal in specificity. It is more active with ethylene diamine than the wild type enzyme and less active with the cognate substrate L-serine.