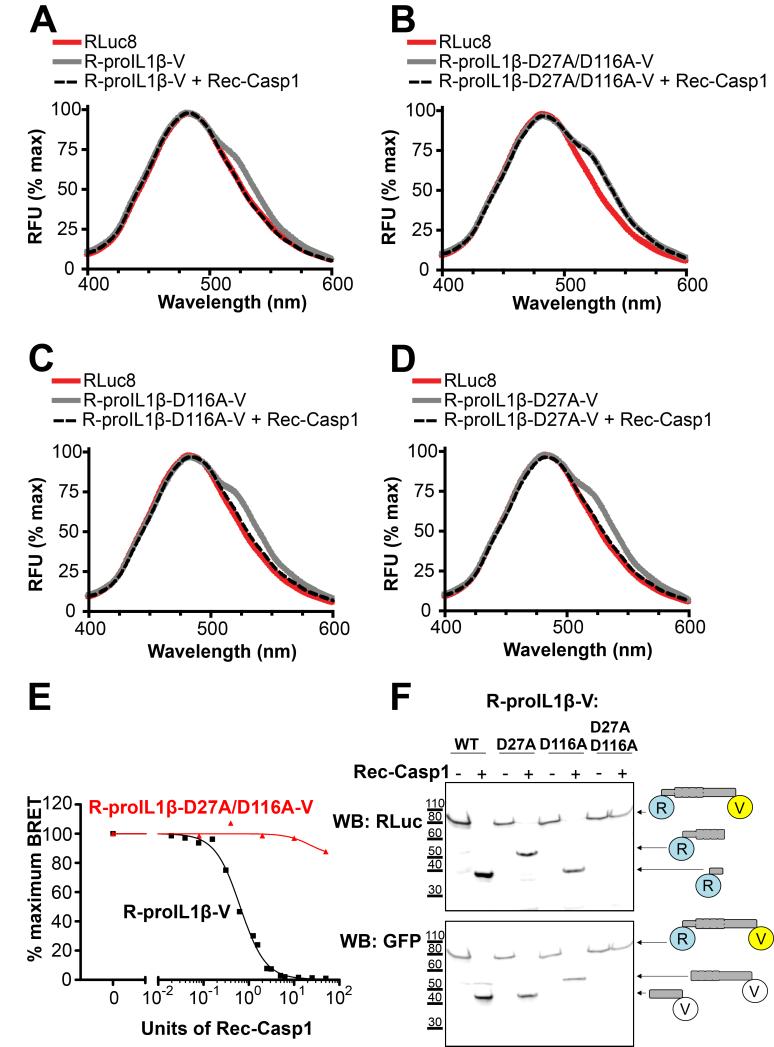
Fig. 2. Response of a BRET sensor to detect IL-1β processing in vitro.
(A) Emission spectra of the R-proIL1β-V sensor. HEK293 cells expressing R-proIL1β-V or RLuc8 were solubilized and incubated for 1 hour at 37°C with or without 50 units of recombinant caspase-1 (Rec-Casp1). After adding coelenterazine h, emission spectra were measured. Note that the shoulder observed at 535 nm for the BRET sensor completely disappeared after addition of caspase-1. (B) Emission spectra of the R-proIL1β-V sensor mutated on the two caspase-1 cleavage sites on residue D27 and D166. Experiments were performed as described in (A) on HEK293 cells expressing mutated R-proIL1β-V (R-proIL1β-D27A/D116A-V). Note that emission spectra of the double mutant superposed before and after incubation with caspase-1. (C and D) Emission spectra of the R-proIL1β-V sensor mutated on one of the two caspase-1 cleavage sites. Experiments were performed as described in (A). Note that shoulder observed at 535 nm for the BRET sensor completely disappear after cleavage by recombinant caspase-1 for the two single mutants. Emission spectra from (A, B, C and D) are representative of three independent experiments. (E) Percentage of BRET signal measured for R-proIL1β-V and R-proIL1β-D27A/D116A-V constructs after incubation with different Units of recombinant caspase-1. Dose response curve is representative of three independent experiments. (F) Western blot analysis of R-proIL1β-V cleavage by Rec-Casp1. HEK293 cells expressing R-proIL1β-V, R-proIL1β-D27A-V, R-proIL1β-D116A-V or R-proIL1β-D27A/D116A-V were treated as described in (A). R-proIL1β-V byproducts were analyzed by Western blot and compared to their expected size as schematized on the right of the blot. The blot shown is representative of four independent experiments.