Abstract
Successful parturition requires the co-ordination of numerous myometrial signalling events to allow for timely and efficient uterine contractions. Late pregnancy and labour onset in humans may be associated with changes in the expression of myometrial proteins implicated in such uterine contractile signal integration. Accordingly, in myometria from non-pregnant women and pregnant women, not in labour or in labour, we examined the content of putative plasmalemmal scaffolding proteins (caveolin-1 and -2) and compared these to the proportions of signal transducing rho-associated kinases (ROK α and β) and contractile filament-associated proteins α-actin, myosin regulatory light chain (MLC20) and h-caldesmon. There was no effect of pregnancy or labour on the proportion of caveolin, ROK β or α-actin. However, pregnancy was associated with a decrease in ROK α and MLC20 such that ROK α : α-actin and MLC20: α-actin ratios were reduced compared to myometria of non-pregnant women. In contrast, h-caldesmon was up-regulated in pregnancy resulting in an elevated h-caldesmon: α-actin ratio. There were, however, no further significant changes in ROK α, MLC20 or h-caldesmon expression with spontaneous or oxytocin-induced labour. These data suggest that the mechanism(s) integrating myometrial signalling events with the onset of human labour does not involve differential alterations of the cellular expressions of caveolins, ROK, α-actin, MLC20 or h-caldesmon.
Keywords: myometrium, pregnancy, labour, signalling molecules, smooth muscle
Introduction
The successful delivery of a fetus and placenta at term requires the timely co-ordination of many signal transducing events to allow for powerful uterine contractions. Such regulation of signalling pathways shifts the balance from a local environment suitable for maintaining relative uterine quiescence, as is beneficial during pregnancy to prevent premature parturition, to a situation primed for powerful uterine contractile effort during labour. Gestational, hormonal and mechanical stimuli may all contribute to altered characteristics of the myometrium. Thus, changes in myometrial physiology towards labour onset at term have been suggested to encompass alterations in the expression of a battery of contractile-associated proteins [1,2]. One may propose, therefore, that in order for uterine contractions to proceed as efficiently as possible, and at term, the expression of molecules involved in the signal integration between excitatory events at the myometrial cell plasma membrane and eventual alteration of intracellular myofilament activation must be tightly controlled. Inappropriate expression of any such molecules may result in altered uterine function contributing to premature delivery or failure of labour to progress at term.
Myometrial contractility is regulated primarily by an elevation of intracellular Ca2+ ([Ca2+]i) resulting, via Ca2+-calmodulin-dependent activation of myosin light chain kinase, in an increased phosphorylation of the regulatory light chains of myosin (MLC20) and enhanced force production [3,4]. An additional mechanism whereby agonists can stimulate myometrial contractility is by sensitising the myofilaments to the activating [Ca2+]i. This occurs in both intact and permeabilised myometrium and in smooth muscle has been associated with corresponding elevations of MLC20 phosphorylation [5–9]. A major pathway for agonist-induced Ca2+-sensitisation, downstream of receptor occupancy, is activation of the small G-protein rhoA and its effecter molecule rho-associated kinase (ROK). ROK stimulation has been shown to result in altered myosin light chain phosphatase activity thereby enhancing net phosphorylated MLC20 and force production [10, 11]. Consequently, pharmacological inhibition of ROK with Y-27632 in intact and permeabilised tissues has been found to inhibit agonist-mediated contractions and MLC20 phosphorylation [11–16].
A further component of uterine contractility, that has been suggested to be a key factor in Ca2+-sensitisation of smooth muscle myofilament activation, is the agonist-mediated relocalisation of rhoA and ROK from the cytosol to the plasma membrane [17]. A major consideration, therefore, is the nature of the membrane structure(s) that co-ordinate the plasmalemmal recruitment of downstream signalling molecules such as ROK. Electron micrography of uterine smooth muscle shows the membrane to consist of periodic electron dense material - the dense plaques where actin filaments insert into the membrane - interspersed with rows of Ω-shaped invaginations known as caveolae [17]. Diverse cellular functions for caveolae have been proposed that, notably, include signal transduction co-ordination [18–20]. Caveolins are a family of proteins integral to the formation of caveolae that, in vitro, interact with a variety of signal transducing molecules including PKCα, Raf, MAP kinase, receptor-dependent tyrosine kinases and rhoA [21] and have been implicated in a variety of cell signalling functions in health and disease. In isolated uterine smooth muscle cells, introduction of peptide derived from a cytoplasmic region of caveolin-1 prevents agonist-mediated relocalisation of rhoA [17]. Such findings have led to the suggestion that caveolins may act as scaffolds for the assembly of signalling complexes at the plasma membrane. Therefore, caveolae, as a result of the properties of caveolins, may be specialised plasmalemmal regions involved in the co-ordination of uterine receptor-coupled signalling events.
Several studies have assessed in human myometrium the transcriptional or translational changes occurring in expression of molecules that influence uterine quiescence [22–24]. In contrast, there is far less comparative information on the effect of pregnancy and labour on myometrial expression of proteins implicated in signalling pathways leading to direct contractile activation; that which is available for individual proteins is often contradictory [25, 26]. Accordingly, in the present study, we have assessed if pregnancy or labour was accompanied by altered expression of plasmalemmal scaffolding proteins (caveolin-1 and caveolin-2), intracellular stimulatory signalling molecules (ROKα and ROKβ) and contractile thick- (regulatory myosin light chain (MLC20)) and thin-filament-associated proteins (α-actin and h-caldesmon) that may participate in the integration of excitatory uterine contractile signals [17,18]. The expressions of each protein of interest were examined in the same samples of myometria isolated from non-pregnant women, term non-labouring pregnant women and term pregnant women in spontaneous or oxytocin-induced labour.
Methods
Tissue collection
Myometrial biopsies were obtained, following written informed consent according to local ethical committee guidelines, from non-pregnant women undergoing hysterectomy (NP) for benign gynaecologic conditions such as uterine bleeding and dysmenorrhoea or women undergoing Caesarean section at term (37–39 weeks) not in labour (PNL), in spontaneous labour (SL) or in oxytocin-induced labour (OT) in compliance with the World Medical Association Declaration of Helsinki. The tissue was collected in Krebs solution (154mM NaCl, 5.4mM KCl, 1.2mM MgSO4, 1.6mM CaCl2, 5.5mM glucose, 10mM MOPS, pH 7.4), in a sterile universal tube, and stored on ice during transfer from the operating theatre to the laboratory. Strips, approximately 7mm3, were dissected from biopsies and snap frozen in liquid N2 before storing at −80°C until use. 10–15mg of frozen tissue was homogenised at 4°C until completion in clean Duall glass tissue grinders in ~250 μl of Homogenisation Buffer (10% glycerol (v/v), 4% sodium dodecyl sulphate (SDS; w/v), 1% Triton X-100 (v/v), 20mM MOPS, pH 7.0, 10mM dithiothreitol, 20mM α-glycerophosphate, 5.5 μM leupeptin, 5.5 μM pepstatin, 0.0205 TIU/ml aprotinin, 20 μM polymethylsulfonylfluoride). Each homogenate was centrifuged at 13000 x g for 5 minutes, the supernatants decanted and the protein concentration of each sample estimated using Bio-Rad DC Protein Assay reagents (Bio-Rad Laboratories Ltd, Hemel Hempstead, Hertfordshire, UK) and construction of a bovine serum albumin (BSA) standard curve. BSA standard absorbances (750nm) were analysed in duplicate whilst samples of unknown protein concentration were analysed in quintuplicate. Following the addition of x2 Laemmli Sample buffer (4% SDS (w/v), 20% glycerol (v/v), 10% 2-mercaptoethanol (v/v), 0.004% bromphenol blue (w/v), 0.125M Tris-HCl, pH 6.8) in a 1:1 ratio, the samples were heated at 95°C for 10 minutes, and subsequently stored at −20°C until use.
SDS-Electrophoresis was performed through the use of the Bio-Rad Mini-Protean II electrophoresis system. A discontinuous polyacrylamide gel system was used consisting of a 4% stacking gel (0.1% SDS (w/v), 0.05% ammonium persulfate, 1:2000 TEMED, 0.125M Tris-HCl, pH 6.8) and 10% separating gel (0.1% SDS (w/v), 0.05% ammonium persulfate, 1:2000 TEMED, 0.375M Tris-HCl, pH 8.8). Following loading of tissue homogenates, gels were electrophoresed in Tris/glycine running buffer at 40mA/gel for 35–40 minutes.
Western blotting was performed through the use of the Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell. The acrylamide gels and methanol-activated PVDF membranes were equilibrated in blotting buffer (25mM Tris, 192mM glycine, 20% methanol, pH 8.3) for 30 minutes. Electrophoretic transfer of protein to the PVDF membrane was performed at 50V/gel for 1.5 hours at room temperature.
Blocked membranes (5% dried milk in Tris-buffered saline (TBS), 1 hour) were incubated overnight at 4°C in TBS with 1% dried milk and one of the following monoclonal primary antibodies: 1:1000 anti-caveolin-1, 1:500 anti-caveolin-2, 1:500 anti-ROKα, 1:250 anti-ROKβ (BD Transduction Laboratories, Oxford, UK), 1:2500 anti-MLC20 or 1:8000 anti-caldesmon (Sigma-Aldrich Company Ltd, Poole, Dorset, UK). Washed membranes were then incubated with 1:2000 anti-mouse IgG, Heavy and Light Chain Specific (Goat) Peroxidase Conjugate (Calbiochem-Novabiochem Ltd, Nottingham, UK) secondary antibody for 1 hour at room temperature. Membrane-bound antibody was stained with enhanced chemiluminescent substrate ((ECL), Super Signal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA) and visualized on X-OMAT AR imaging film (Kodak, Rochester, NY, USA). Membranes were also either (i) cut at appropriate molecular weights following electrophoretic transfer, or (ii) stripped of original primary antibody, and probed with 1:100000 anti-α smooth muscle actin monoclonal antibody (Sigma-Aldrich Company Ltd, UK), incubated with secondary antibody and stained with ECL as above. Predicted molecular weights for antibody-probed proteins were caveolin-1 21kDa, caveolin-2 21kDa, ROKα180kDa, ROKβ160kDa, α-actin 44kDa, MLC20 20kDa and h- caldesmon 130kDa. The anti-ROKβ antibody consistently resulted in the appearance of a doublet: one band of the anticipated molecular weight of 160kDa that corresponded to the band of a known positive control (mouse kidney cell lysate) and another band of molecular weight ~200kDa. The larger band co-localised on stained membranes with myosin heavy chain (205kDa) and is likely to represent a non-specific cross-reactivity with this protein. As such, the lower 160kDa band alone was taken to represent ROKβ.
For relative protein quantifications between conditions (e.g., myometrial α-actin expression in NP versus PNL women), at least two homogenate amounts (5–40 μg/lane) were always compared from the same membrane. This ensured: (i) identical treatment of samples from different conditions throughout the entire detection procedure; (ii) that ECL signals had not saturated and (iii) that any contribution of small pipetting errors between lanes to differences in ECL signal detection and densitometric data quantification was minimised. Protein quantification was determined by densitometric scanning (BIO-RAD GS-700 and Molecular Analyst; Bio-Rad Laboratories Ltd, UK) of imaging films. Comparisons between conditions for any one protein of interest were performed as follows: (i) NP versus PNL. All densitometric raw data values were compared between the two conditions for the comparable amounts of protein loaded, the values meaned and normalised with respect to the NP sample (taken as 1.0). (ii) PNL versus SL or OT. All densitometric raw data values were compared between the three conditions for the comparable amounts of protein loaded, the values meaned and normalised with respect to the PNL sample (taken as 1.0). In addition, an assessment of the amounts of any one protein of interest relative to α-actin was performed as follows: The normalised values for each homogenate loading were obtained for the protein of interest as above and, from the same membrane, for α-actin. On averaging the figures from two separate loadings, values for any change in expression for the chosen protein of interest, and for α-actin, with pregnancy (PNL c.f. NP) or labour (OT or SL c.f. PNL) were obtained. For example: caveolin-1 expression in PNL was assessed as x-fold of NP and α-actin expression in PNL was determined as y-fold of NP. Therefore, for each patient a value of caveolin-1: α-actin expression in PNL versus NP of x-fold/y-fold was obtained. In such a manner, a ratio of the amounts of each protein of interest relative to α-actin for PNL c.f. NP, and for OT or SL c.f. PNL, conditions was calculated.
Materials
Materials other than those mentioned above were purchased from Sigma-Aldrich Company Ltd, UK, except the following: NaCl, CaCl2, NaHCO3, KHPO4, K2EDTA, acetic acid and methanol (VWR International Ltd, Lutterworth, Leicestershire, UK), acrylamide/bis acrylamide and filter paper (BioRad Laboratories Ltd, UK), PVDF membrane (Scheicher & Schuell, Dassel, Germany).
Statistics
Differences in α-actin, and normalised protein: α-actin ratios, between NP and PNL groups were analysed by one sample students t-test. Differences in α-actin, normalised protein: α-actin ratios, between PNL, SL or OT groups were analysed by one-way ANOVA followed by Dunnets post-hoc test. Significance was taken as P<0,05. All results are expressed as mean ± S.E.M. N = number of patients; n = number of times that the individual patient sample sets were electrophoresed and immunoblotted and the resulting data were meaned per ‘N’.
Results
Effect of pregnancy on myometrial protein expression
As illustrated in the representative example of Figure 1A, the proportion of α-actin expressed in tissue homogenates did not change between NP and PNL samples. On average, the α-actin proportion in PNL samples was 0.96±0.06-fold of NP samples (Fig. 1B, N=13). This allowed a comparison of other proteins of interest to be made with α-actin.
Fig. 1.
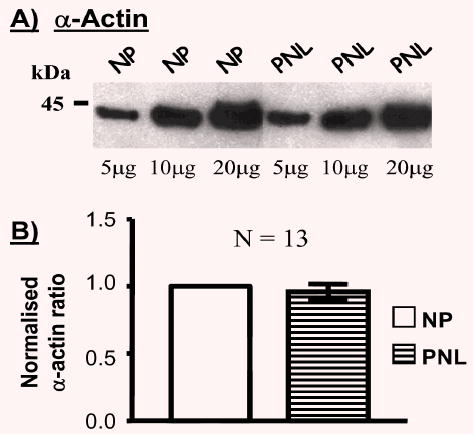
Effect of pregnancy on the relative expressions of α-actin in human myometria. (A) Representative blot illustrating α-actin expression in myometrial homogenates from non-pregnant (NP) women and pregnant women not in labour (PNL). In this diagram, there was no difference in α-actin expression between NP and PNL samples. Densitometric data from the separate protein loadings for NP or PNL conditions (e.g. 5 μg NP compared with 5 μg PNL, 10 μg compared to 10 μg etc) were divided and the values across the different protein loadings meaned to reflect the relative quantities of α-actin between these NP and PNL samples. (B) Subsequently, between patients, all the PNL meaned values were normalised to the corresponding NP values (taken as 1.0) to reveal the overall relationship of α-actin expression between 13 NP and PNL samples.
Caveolin-1 expression, relative to total protein content, was also not altered with pregnancy (Fig. 2A). On average, the caveolin-1 proportion in PNL samples was 0.94±0.06-fold of NP samples (Fig. 2A, N=6). In addition, the same blots and tissues were assessed for the proportion of α-actin content (proportion in PNL samples was 1.05±0.11-fold of NP samples, N=6, Fig. 2B) such that the ratio of caveolin-1: α-actin in PNL samples was also unchanged when compared to NP (0.98±0.14-fold, N=6, Fig. 2C). A similar situation existed for caveolin-2 (Fig. 2D). The proportion in PNL samples was 1.05±0.15-fold of NP tissues (N=7) such that the ratio of caveolin-2: α-actin in PNL samples was 1.04±0.20-fold of that in NP tissues (Fig. 2F).
Fig. 2.
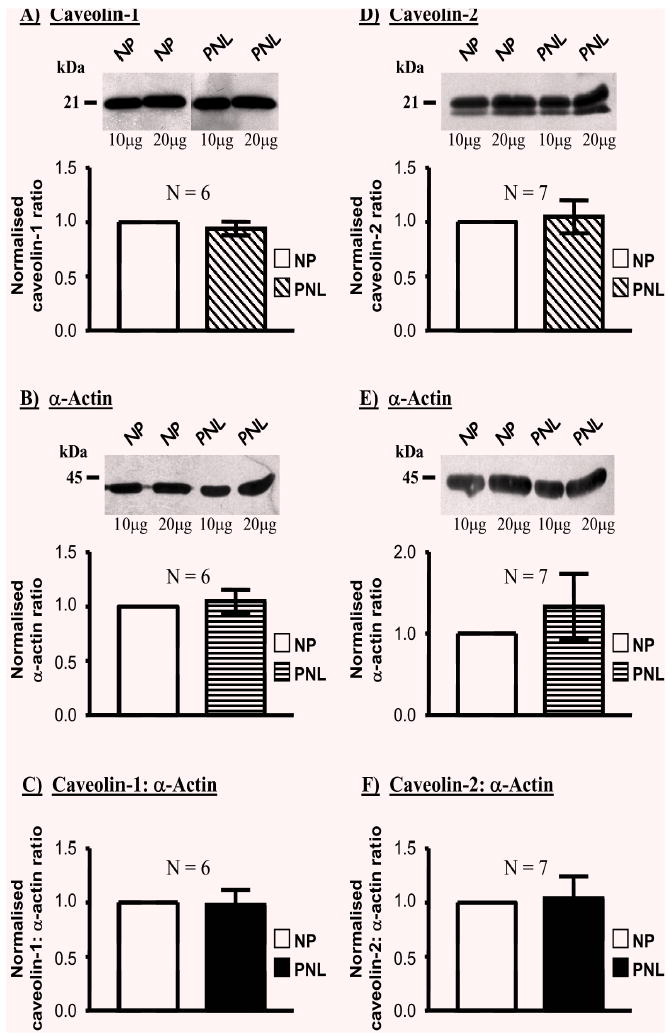
Effect of pregnancy on the relative expressions of cave-olin-1 and -2, relative to α-actin, in human myometria. Myometrial homogenates from pregnant women not in labour (PNL) expressed similar amounts of caveolin-1 (A) and caveolin-2 (D), and α-actin (B & E) as tissues from non-pregnant (NP) women. Consequently, there were no differences in the caveolin-1: α-actin (C) or cave-olin-2: α-actin (F) ratios in myometria from NP versus PNL.
ROKβ(Fig. 3A) content was not significantly different between myometria of pregnant and non-pregnant women (the proportion in PNL tissues was 1.20±0.20-fold of NP samples, N=4). Again, this resulted in ROKβ: α-actin ratios in PNL samples that were unchanged compared to NP situations (1.28±0.17-fold (Fig. 3C) respectively). However, the expression of ROKα(Fig. 3D) was significantly changed in PNL myometrial samples compared to NP tissues (0.66±0.05-fold, N=6) such that the ratio of ROKα: α-actin in PNL samples was 0.72±0.06-fold (Fig. 3F) of that in NP tissues.
Fig. 3.
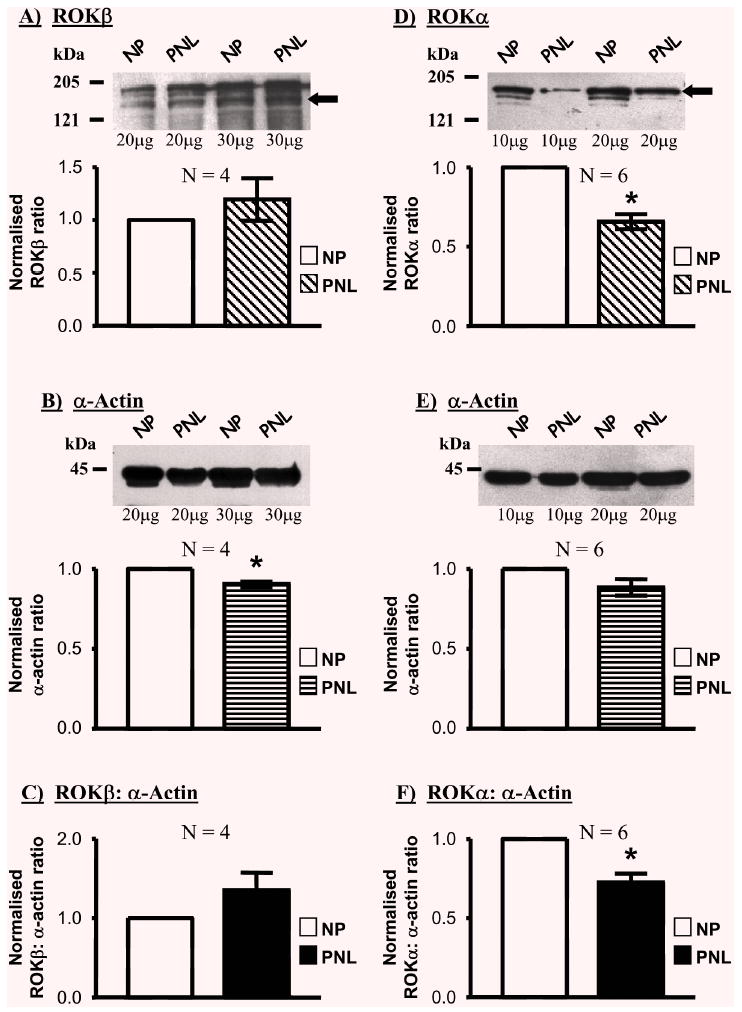
Relative expressions of ROKβ, ROKα and α-actin in NP and PNL myometrial samples. Myometrial homogenates from pregnant women not in labour (PNL) expressed a similar amount of ROKβ (A; band denoted by the arrow) relative to α-actin content (B) as tissues from non-pregnant (NP) women. There was, thus, no difference in the ROKβ: α-actin ratio between the two conditions (C). ROKα expression (D; band denoted by the arrow) relative to α-actin (E & F), reduced significantly during pregnancy, c.f. non-pregnant myometria.
Expression of the thick filament-associated MLC20 (Fig. 4A) was also reduced in PNL samples (0.52±0.03-fold, N=4) than in NP tissues. This, in turn, resulted in ratios of MLC20: α-actin being significantly less in PNL samples (0.58±0.04-fold) compared to NP (Fig. 4C). In contrast, the expression of the thin filament-associated h-caldesmon (Fig. 4D) increased in PNL to 2.61±0.41-fold of the NP state; hence the h-caldesmon: α-actin ratio was significantly elevated compared to NP samples (2.68±0.46-fold, N=6, Fig. 4F).
Fig. 4.
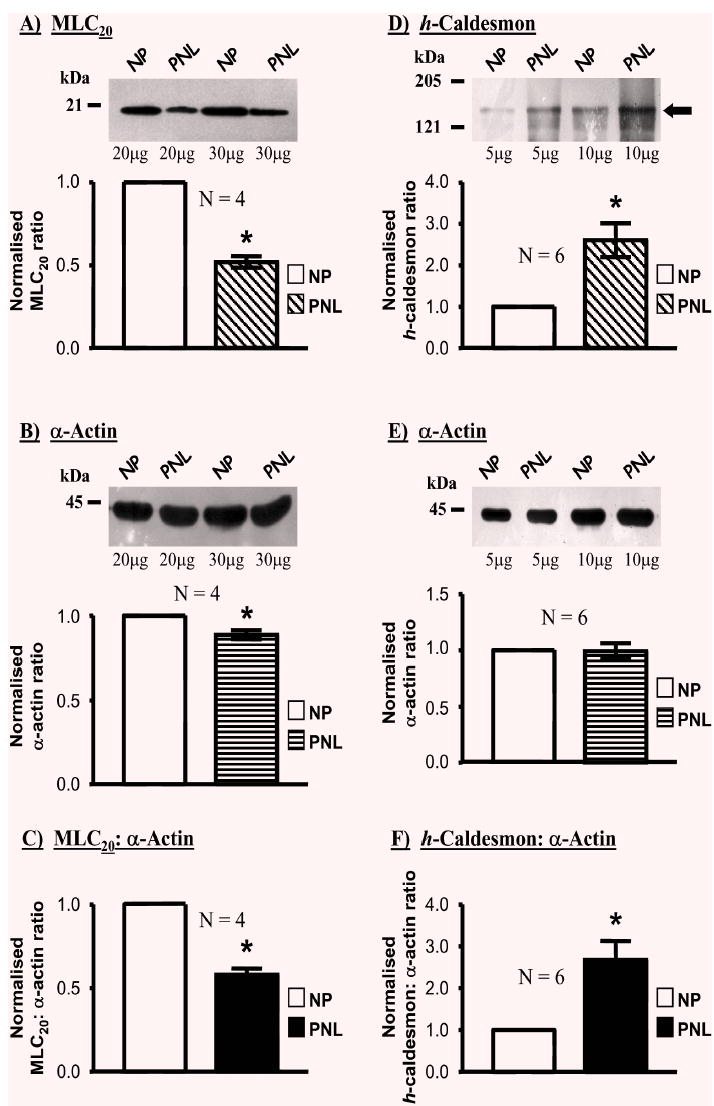
Relative expressions of the contractile filament-associated proteins MLC20, h-caldesmon and α -actin in NP and PNL myometrial samples. Myometrial homogenates from pregnant women not in labour (PNL) contained significantly less MLC20 (A) than non-pregnant women (NP) relative α-actin content (B). Consequently, the MLC20: α-actin ratio in myometria of PNL women was lower than those of NP women (C). An increase in h-caldesmon expression relative to α-actin content was evident in pregnancy, c.f. non-pregnant myometria (D & E; band denoted by the arrow). This resulted in a significant rise in the h-caldesmon: α-actin ratio in PNL versus NP patients (F).
Effect of labour on myometrial protein expression
A comparison of myometrial protein expression in samples from pregnant women not in labour (PNL) with women in spontaneous labour (SL) or oxytocin-induced labour (OT) was performed. As illustrated in the representative blot and meaned data of Fig. 5, the proportion of α-actin was unaltered being 0.93±0.08-fold in SL samples and 1.02±0.03-fold in OT tissues compared to that in PNL situations (N=4, n=20). This again allowed a comparison to α-actin of labour-dependent changes in expression of other proteins of interest.
Fig. 5.
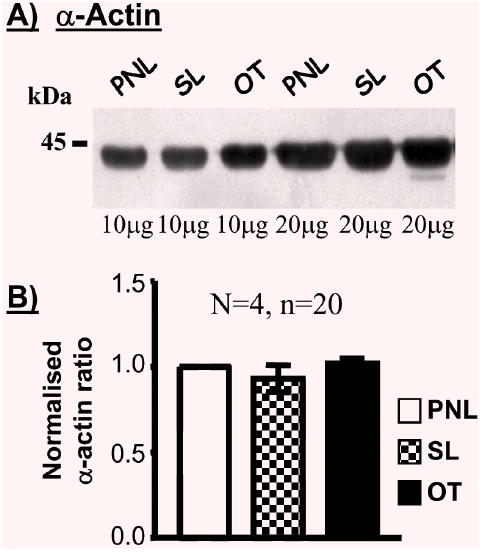
The expressions of α-actin in myometria of non-labouring and labouring women. (A) Representative Western blot illustrating the content of α-actin in myometrial tissue homogenates from pregnant women not in labour (PNL) and pregnant women in spontaneous (SL) or oxytocin-induced (OT) labour. (B) Meaned data demonstrating no effect of labour on the expression of α-actin.
Representative blots of caveolin-1, caveolin-2, ROKα, ROKβ, MLC20 or h-caldesmon are shown in Fig. 6 and the ratios of each protein relative to α-actin illustrated in Fig. 7. Labour onset was not associated with proportional changes in myometrial expressions of caveolin-1 (0.87±0.11-fold in SL and 0.93±0.09-fold in OT, N=4) and caveolin-2 (0.91±0.02-fold in SL and 0.90±0.06-fold in OT, N=4), ROKα(1.03±0.07-fold in SL and 1.05±0.07-fold in OT, N=4) or ROKβ(0.92±0.09-fold in SL and 0.96+0.11-fold in OT, N=4), MLC20 (0.99±0.16-fold in SL and 0.87±0.12-fold in OT, N=4) or h-caldesmon (1.38±0.28-fold in SL and 0.91±0.16-fold in OT, N=4) relative to PNL (Fig. 6). The caveolin-1: α-actin (SL 0.85±0.10-fold; OT 0.86±0.12-fold), caveolin-2: α-actin (SL 0.93±0.05-fold; OT 0.93±0.11-fold), ROKα: α-actin (SL 1.13±0.07-fold; OT 1.06±0.08-fold), ROKβ: α-actin (SL 1.00±0.03-fold; OT 1.09±0.17-fold), MLC20: α-actin (SL 1.11±0.21-fold; OT 0.84±0.11-fold) and h-caldesmon: α-actin (SL 1.77±0.33-fold; OT 0.99±0.15-fold) ratios were invariant between myometrial samples from term non-labouring and labouring women (Fig. 7).
Fig. 6.
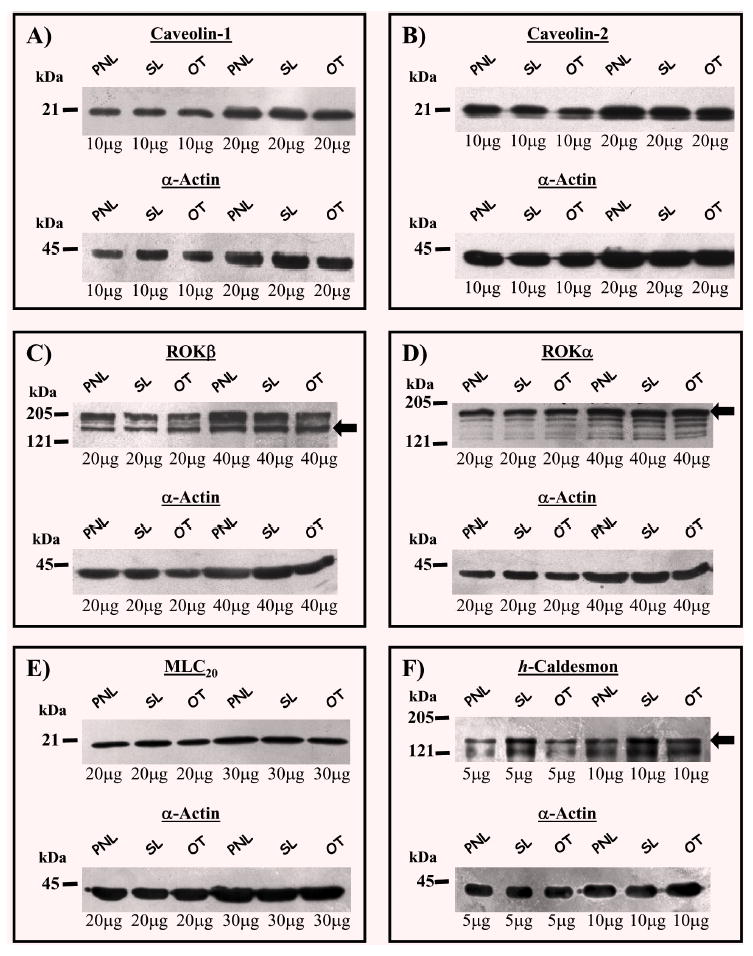
The expressions of caveolin-1, caveolin-2, ROKβ, ROKα, MLC20, h-caldesmon and α-actin in myometria of non-labouring and labouring women. Representative Western blots illustrating the content of caveolin-1, caveolin-2, ROKα, ROKβ, MLC20 and h-caldesmon (bands denoted by arrows) in myometrial tissue homogenates from pregnant women not in labour (PNL) and pregnant women in spontaneous (SL) or oxytocin-induced (OT) labour. For each protein the corresponding α-actin signal from the same membrane is illustrated.
Fig. 7.
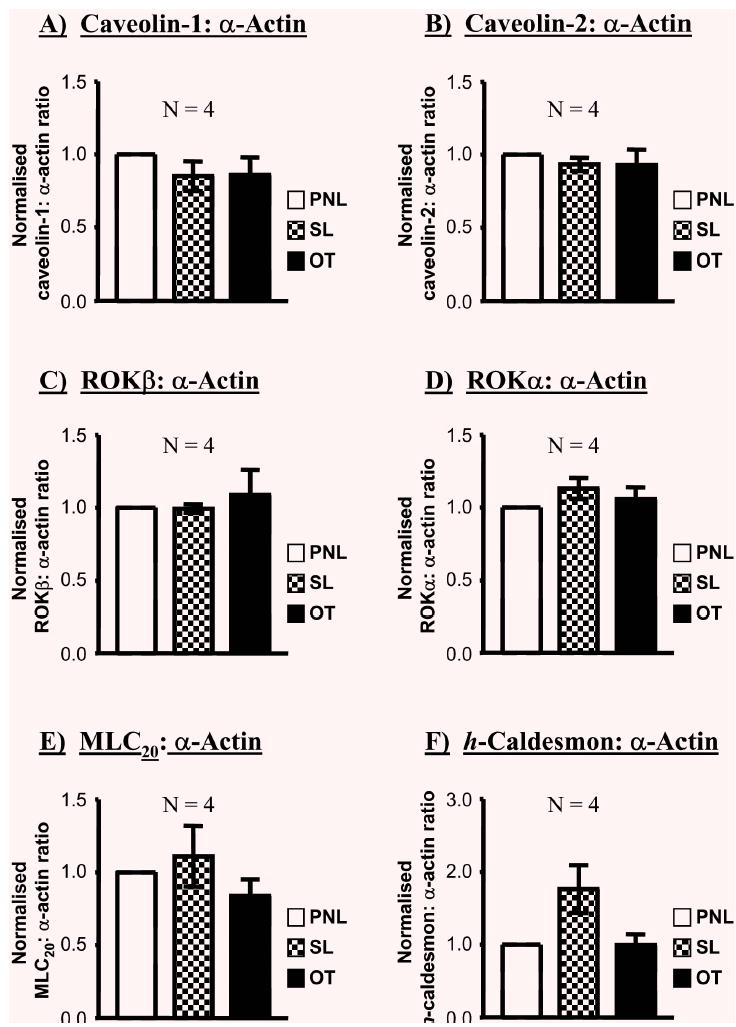
The effect of labour on the myometrial expressions of caveolin-1, caveolin-2, ROKβ, ROKα, MLC20 and h-caldesmon relative to α-actin. When expressed as a ratio to α-actin, there were no significant differences in myometrial expression of each protein between pregnant women not in labour (PNL) and pregnant women in spontaneous (SL) or oxytocin-induced (OT) labour.
Discussion
In this study we have observed the effects of pregnancy, and spontaneous or oxytocin-induced labour, on the human myometrial expression of several proteins key to the integration of signal-contraction coupling in the uterus. We find that pregnancy is associated with a down-regulation of MLC20 and ROKα but that the relative expressions of α-actin, caveolin-1 and -2 and ROKβ were unaltered. In contrast, in the same samples, h-caldesmon was elevated. Furthermore, the onset of spontaneous labour, or the induction of labour with oxytocin, was not associated with changes in expression of any of these myometrial proteins when compared to samples from pregnant women at term not in labour.
Quantification of relative protein expressions
The methodological approach reported herein to examine myometrial protein expression changes was undertaken in an effort to minimise any errors in the quantification of protein expression between different tissues and conditions. Firstly, all samples examined on the same gels were protein matched and at least two different protein homogenate loadings were analysed for each sample. This ensured that ECL signals for each protein of interest had not saturated (which could otherwise have skewed the data analysis) and, because the assessment of protein expression between conditions was an average of multiple data points per gel, it also reduced the contribution of any unforeseen small pipetting errors between lanes. Secondly, the reporting of a true change in myometrial cellular protein expression with pregnancy, or labour, necessitates a comparison with a control protein that shows no changes between conditions. Word et al. [27] reported that the myometrial expression of total actin, relative to total homogenate protein, was unchanged in pregnancy. In the present study, we have confirmed this also to be the case for the α-actin isoform. We also found that α-actin expression remained unaltered in homogenates from women in spontaneous or oxytocin-induced labour compared to women at term not in labour. This allowed a calculation of the content of our other proteins of interest to be made relative to the invariant α-actin expression on each gel. In turn, this served as an additional internal control to ensure equable protein loadings between lanes and to minimise against any operator errors in the entire quantification procedure. Finally, all the proteins of interest were analysed from the same sample sets; thus, α-actin served as a constant reference point within all the samples for comparison with the other proteins of interest.
Expression of caveolin-1, -2, ROK, MLC20 and h-caldesmon in human myometria with pregnancy and labour onset
The expressions of caveolin-1 or -2, relative to total homogenate or to α-actin, did not change between NP and PNL women. In addition, myometrial caveolin levels were unaffected by the onset of spontaneous or oxytocin-mediated labour. Caveolins are integral protein components of plasmalemmal membrane invaginations termed caveolae such that caveolin expression appears essential to caveolae formation [28]. In human myometrium, however, the area of surface membrane occupied by caveolae is unchanged in myometria of non-pregnant and term pregnant woman (Riley and Taggart, unpublished data), and doesn’t differ between women not in labour and those in labour [29], findings that are consistent with the lack of change in expression of caveolins-1 and -2 with pregnancy or labour reported here.
Equally, the expression of ROKβ was unchanged with pregnancy. However, the expression of ROK α was significantly reduced in PNL women compared to NP women. This is in contrast to an earlier report qualitatively assessing an increase in ROK expression at term [25] in which there was a suggestion of an increase in ROKβ expression with pregnancy. The reasons for this discrepancy are unclear but may reflect (i) the larger number of samples quantified with respect to an invariant protein in the present study and (ii) the use of different commercially available anti-ROK antibodies. A recent preliminary study supports our findings of myometrial ROKβ protein expression not changing with pregnancy [26]. ROK proteins are implicated in agonist-mediated contractions of animal and human myometrium [13–15,18], in particular agonist-dependent Ca2+-sensitisation of contraction. One consequence, therefore, of ROK expression not being elevated with pregnancy may be to limit premature activation of this excitatory contractile signalling pathway. However, a prominent role of ROK activation in human uterine contractility has also been questioned [16]. There were no further changes in ROKα or ROKβ expression with labour onset indicating that parturition was not associated with an up-regulation of myometrial ROK expression.
There was a marked reduction in the myometrial expression of MLC20 with pregnancy that was not further changed by the onset of labour. Ca2+-dependent activation of MLC20 phosphorylation is a crucial step in the initiation of uterine contractions [27]. However, pregnancy is also associated with reductions in the dynamics and extent of myometrial MLC20 phosphorylation whilst force generating capacity increases [27,30]. These effects may partly be explicable by the reduced MLC20 expression with pregnancy observed here and may assist in the maintenance of relative uterine quiescence. These results also imply that uterine activation at term is likely to involve Ca2+-dependent pathways in addition to MLC20 phosphorylation [31].
Of the myometrial proteins examined in this study only h-caldesmon exhibited up-regulation with pregnancy. This is consistent with that previously reported for both human and rat myometrium [22,27,32]. h-Caldesmon is a thin filament-associated protein that inhibits acto-myosin MgATPase activity [33]. The increased expression of h-caldesmon with pregnancy has been suggested, therefore, to contribute to the maintenance of gestation. Notably, h-caldesmon expression was not further altered by the onset of labour.
Physiological significance of the findings
There is an emerging body of evidence suggesting that human pregnancy, and labour onset, is associated with translational changes in many molecules whose main influence is to limit uterine activity [22,23,34–36]. As ROKα and MLC20 are key proteins implicated in smooth muscle contractile activation, their decreased expression with pregnancy reported here, coupled with an enhanced expression of h-caldesmon, may also contribute to this adaptive situation before parturition. Of course, myometrial cells undergo significant hypertrophy with pregnancy yet the content of contractile proteins per cross sectional area remains constant [27]. This implies that the actual cellular content of many proteins, including actin and caveolins, must increase with pregnancy but that this occurs in proportion to total protein content. Thus, to simply maintain a constant proportion of a particular protein relative to total cellular protein content is, in itself, an important adaptation to the physiological stresses imposed on the uterus during pregnancy. This will also ensure the requisite presence of components of the contractile response if parturition occurs, at least in part, as a result of the release of inhibitory influences [23,24,34–36]. The fact that no further changes in the proportions of ROK, MLC20, and caldesmon, nor of α-actin, caveolin-1 and caveolin-2, were observed with the onset of labour suggests that differential translational regulation of these molecules is not a necessity for the integrated uterine contractile response of parturition. If these molecules do play a role in the scaffolding, transduction and activation of uterine contractile signals during labour then it is likely to arise from a regulation of molecular activity, for example by altered protein-protein interactions and/or post-translational modifications. Whilst these remain to be explored in future studies of human myometrium, it is noteworthy that support for such a concept comes from recent observations in animal experiments in which labour was accompanied by significant post-translational changes in both h-caldesmon and MLC20 [32].
Acknowledgments
This work was supported by Tommy’s, the baby charity and The Wellcome Trust.
References
- 1.Challis J.R.G., Lye S.J., Parturition. In: Knobil E., Neill JD., eds., The Physiology of Reproduction, edn.5, Raven Press/New York, pp. 985–1031, 1994
- 2.Dalrymple A, Slater DM, Poston L, Tribe RM. Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor, and interleukin-1. J Clin Endocrinol Metab. 2004;89:1291–1300. doi: 10.1210/jc.2003-031428. [DOI] [PubMed] [Google Scholar]
- 3.Taggart MJ, Menice CB, Morgan KG, Wray S. Effect of metabolic inhibition on intracellular Ca2+, phosphorylation of myosin regulatory light chain and force in rat smooth muscle. J Physiol. 1997;499:485–496. doi: 10.1113/jphysiol.1997.sp021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Word RA, Casey ML, Kamm KE, Stull JT. Effects of cGMP on [Ca2+]i, myosin light chain phosphorylation, and contraction in human myometrium. Am J Physiol. 1991;260:C861–867. doi: 10.1152/ajpcell.1991.260.4.C861. [DOI] [PubMed] [Google Scholar]
- 5.Taggart MJ, Wray S. Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activation: gestational dependence in isolated rat uterus. J Physiol. 1998;511:133–144. doi: 10.1111/j.1469-7793.1998.133bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YH, Hwang MK, Morgan KG, Taggart MJ. Receptor-coupled contractility of uterine smooth muscle: from membrane to myofilaments. Exp Physiol. 2001;86:283–288. doi: 10.1113/eph8602184. [DOI] [PubMed] [Google Scholar]
- 7.Sward K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J Physiol. 2000;522:33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujihara H, Walker LA, Gong MC, Lemichez E, Boquet P, Somlyo AV, Somlyo AP. Inhibition of RhoA translocation and calcium sensitization by in vivo ADP-ribosylation with the chimeric toxin DC3B. Mol Biol Cell. 1997;8:2437–2447. doi: 10.1091/mbc.8.12.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucius C, Arner A, Steusloff A, Troschka M, Hofmann F, Aktories K, Pfitzer G. Clostridium difficile toxin B inhibits carbachol-induced force and myosin light chain phosphorylation in guinea-pig smooth muscle: role of Rho proteins. J Physiol. 1998;506:83–93. doi: 10.1111/j.1469-7793.1998.083bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta Physiol Scand. 1998;164:483–493. doi: 10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- 11.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 12.Sward K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep. 2003;5:66–72. doi: 10.1007/s11906-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 13.Oh JH, You SK, Hwang MK, Ahn DS, Kwon SC, Taggart MJ, Lee YH. Inhibition of Rho-associated kinase reduces MLC20 phosphorylation and contractility of intact myometrium and attenuates agonist-induced Ca2+ sensitization of force in permeabilized rat myometrium. J Vet Med Sci. 2003;65:43–50. doi: 10.1292/jvms.65.43. [DOI] [PubMed] [Google Scholar]
- 14.Moran CJ, Friel AM, Smith TJ, Cairns M, Morrison JJ. Expression and modulation of Rho kinase in human pregnant myometrium. Mol Hum Reprod. 2002;8:196–200. doi: 10.1093/molehr/8.2.196. [DOI] [PubMed] [Google Scholar]
- 15.Woodcock NA, Taylor CW, Thornton S. Effect of an oxytocin receptor antagonist and rho kinase inhibitor on the [Ca2+]i sensitivity of human myometrium. Am J Obstet Gynecol. 2004;190:222–228. doi: 10.1016/s0002-9378(03)00925-6. [DOI] [PubMed] [Google Scholar]
- 16.Kupittayanant S, Burdyga T, Wray S. The effects of inhibiting Rho-associated kinase with Y-27632 on force and intracellular calcium in human myometrium. Pflugers Arch. 2001;443:112–114. doi: 10.1007/s004240100668. [DOI] [PubMed] [Google Scholar]
- 17.Taggart MJ, Leavis P, Feron O, Morgan KG. Inhibition of PKC and rhoA translocation in differentiated smooth muscle by a caveolin scaffolding domain peptide. Exp Cell Res. 2000;258:72–81. doi: 10.1006/excr.2000.4891. [DOI] [PubMed] [Google Scholar]
- 18.Taggart MJ. Smooth muscle excitation-contraction coupling: a role for caveolae and caveolins? News Physiol Sci. 2001;16:61–65. doi: 10.1152/physiologyonline.2001.16.2.61. [DOI] [PubMed] [Google Scholar]
- 19.Feron O. Endothelial nitric oxide synthase expression and its functionality. Curr Opin Clin Nutr Metab Care. 1999;2:291–296. doi: 10.1097/00075197-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Parton RG. Caveolae--from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol. 2003;4:162–167. doi: 10.1038/nrm1017. [DOI] [PubMed] [Google Scholar]
- 21.Gingras D, Gauthier F, Lamy S, Desrosiers RR, Beliveau R. Localization of RhoA GTPase to endothelial caveolae-enriched membrane domains. Biochem Biophys Res Commun. 1998;247:888–893. doi: 10.1006/bbrc.1998.8885. [DOI] [PubMed] [Google Scholar]
- 22.Cornwell TL, Li J, Sellak H, Miller RT, Word RA. Reorganization of myofilament proteins and decreased cGMP-dependent protein kinase in the human uterus during pregnancy. J Clin Endocrinol Metab. 2001;86:3981–3988. doi: 10.1210/jcem.86.8.7727. [DOI] [PubMed] [Google Scholar]
- 23.MacDougall MW, Europe-Finner GN, Robson SC. Human myometrial quiescence and activation during gestation and parturition involve dramatic changes in expression and activity of particulate type II (RII ) protein kinase A holoenzyme. J Clin Endocrinol Metab. 2003;88:2194–2205. doi: 10.1210/jc.2002-021862. [DOI] [PubMed] [Google Scholar]
- 24.Chanrachakul B, Matharoo-Ball B, Turner A, Robinson G, Broughton-Pipkin F, Arulkumaran S, Khan RN. Reduced expression of immunoreactive 2-adrenergic receptor protein in human myometrium with labor. J Clin Endocrinol Metab. 2003;88:4997–5001. doi: 10.1210/jc.2003-030692. [DOI] [PubMed] [Google Scholar]
- 25.Moore F, Da Silva C, Wilde JI, Smarason A, Watson SP, Lopez Bernal A. Up-regulation of p21- and RhoA-activated protein kinases in human pregnant myometrium. Biochem Biophys Res Commun. 2000;269:322–326. doi: 10.1006/bbrc.2000.2290. [DOI] [PubMed] [Google Scholar]
- 26.Friel AM, Ravikumar N, Smith TJ, Morrison JJ. RhoA and ROCK-I protein levels in human myometrium during pregnancy. J Soc Gynecol Invest. 2004;11:227A. doi: 10.1016/j.jsgi.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Word RA, Stull JT, Casey ML, Kamm KE. Contractile elements and myosin light chain phosphorylation in myometrial tissue from nonpregnant and pregnant women. J Clin Invest. 1993;92:29–37. doi: 10.1172/JCI116564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, Zurzolo C. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J Cell Biol. 1998;140:617–626. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciray HN, Guner H, Hakansson H, Tekelioglu M, Roomans GM, Ulmsten U. Morphometric analysis of gap junctions in nonpregnant and term pregnant human myometrium. Acta Obstet Gynecol Scand. 1995;74:497–504. doi: 10.3109/00016349509024378. [DOI] [PubMed] [Google Scholar]
- 30.Obayashi HY, Word RA. Relationship between protein kinase C, caldesmon phosphorylation, and force generation in human myometrium during pregnancy. J Soc Gynecol Invest. 2003;10:130A. [Google Scholar]
- 31.Ozaki H, Yasuda K, Kim YS, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol. 2003;140:1303–1312. doi: 10.1038/sj.bjp.0705552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Je HD, Malek S, Morgan KG. ERK1/2-mediated phosphorylation of myometrial caldesmon during pregnancy and labor. Am J Physiol Regul Integr Comp Physiol. 2003;284:R192–199. doi: 10.1152/ajpregu.00290.2002. [DOI] [PubMed] [Google Scholar]
- 33.Marston S. Calcium ion-dependent regulation of uterine smooth muscle thin filaments by caldesmon. Am J Obstet Gynecol. 1989;160:252–257. doi: 10.1016/0002-9378(89)90131-2. [DOI] [PubMed] [Google Scholar]
- 34.Europe-Finner GN, Phaneuf S, Mardon HJ, Lopez Bernal A. Human myometrial G s-small (with serine) and Gs-large (with serine) messenger ribonucleic acid splice variants promote the increased expression of 46- and 54-kilodalton G s protein isoforms in pregnancy and their down-regulation during labor. J Clin Endocrinol Metab. 1996;81:1069–1075. doi: 10.1210/jcem.81.3.8772578. [DOI] [PubMed] [Google Scholar]
- 35.Chanrachakul B, Matharoo-Ball B, Turner A, Robinson G, Broughton-Pipkin F, Arulkumaran S, Khan RN. Immunolocalization and protein expression of the α-subunit of the large-conductance calcium-activated potassium channel in human myometrium. Reprod. 2003;126:43–48. doi: 10.1530/rep.0.1260043. [DOI] [PubMed] [Google Scholar]
- 36.Matharoo-Ball B, Ashford ML, Arulkumaran S, Khan RN. Down-regulation of the α- and β-subunits of the calcium-activated potassium channel in human myometrium with parturition. Biol Reprod. 2003;68:2135–2141. doi: 10.1095/biolreprod.102.010454. [DOI] [PubMed] [Google Scholar]


