Abstract
Amprenavir is one of six protease inhibitors presently approved for clinical use in the therapeutic treatment of AIDS. Biochemical and clinical studies have shown that, unlike other inhibitors, Amprenavir is severely affected by the protease mutation I50V, located in the flap region of the enzyme. TMC-126 is a second-generation inhibitor, chemically related to Amprenavir, with a reported extremely low susceptibility to existing resistant mutations including I50V. In this paper, we have studied the thermodynamic and molecular origin of the response of these two inhibitors to the I50V mutation and the double active-site mutation V82F/I84V that affects all existing clinical inhibitors. Amprenavir binds to the wild-type HIV-1 protease with high affinity (5.0 × 109 M−1 or 200 pM) in a process equally favored by enthalpic and entropic contributions. The mutations I50V and V82F/I84V lower the binding affinity of Amprenavir by a factor of 147 and 104, respectively. TMC-126, on the other hand, binds to the wild-type protease with extremely high binding affinity (2.6 × 1011 M−1 or 3.9 pM) in a process in which enthalpic contributions overpower entropic contributions by almost a factor of 4. The mutations I50V and V82F/I84V lower the binding affinity of TMC-126 by only a factor of 16 and 11, respectively, indicating that the binding affinity of TMC-126 to the drug-resistant mutants is still higher than the affinity of Amprenavir to the wild-type protease. Analysis of the data for TMC-126 and KNI-764, another second-generation inhibitor, indicates that their low susceptibility to mutations is caused by their ability to compensate for the loss of interactions with the mutated target by a more favorable entropy of binding.
Keywords: HIV-1 protease, drug resistance, HIV-1 protease inhibitors, isothermal titration calorimetry, Amprenavir, TMC-126
A major limiting factor in the treatment of the HIV-1 infection is the emergence of viral strains that show resistance to protease inhibitors (Ho et al. 1994; Kaplan et al. 1994; Condra et al. 1995; Roberts 1995; Hong et al. 1996; Tisdale 1996; Ala et al. 1997; Jadhav et al. 1997; Todd et al. 2000). It has been estimated that one out of every seven new infections in America is caused by viral strains resistant to at least one type of inhibitor. The loss of sensitivity to protease inhibitors usually occurs because the resistant viral strains encode for protease molecules containing specific amino acid mutations that lower the affinity for the inhibitors, yet maintain sufficient affinity for the substrate; that is, the impact of these mutations is more pronounced on inhibitor binding than on substrate binding.
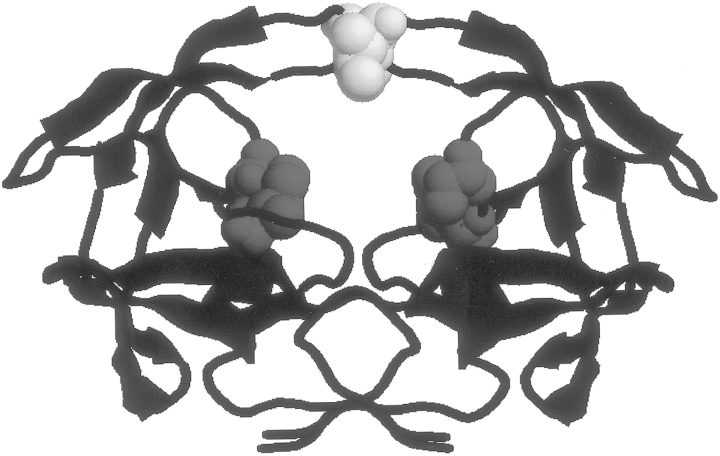
Previously, we reported the thermodynamic analysis of the binding of four protease inhibitors in clinical use (Indinavir, Saquinavir, Nelfinavir, and Ritonavir; Todd et al. 2000) and a second-generation inhibitor (KNI-764; Velazquez-Campoy et al. 2001a) to the wild-type and the drug-resistant double mutant V82F/I84V, known to affect all clinical inhibitors including Amprenavir (Markowitz et al. 1995; Ala et al. 1998; Klabe et al. 1998). The V82F/I84V mutation is located at the edges of the active site (Fig. 1 ▶) in a well-structured and stable region of the protease (Todd et al. 1998); its main effect on the binding affinity of inhibitors appears to be related to a change in the three-dimensional geometry of the binding site because its polarity and charge remain unchanged (Todd et al. 2000). Unlike other clinical inhibitors, Amprenavir is severely affected by the single mutation I50V (Kim et al. 1995; Pazhanisamy et al. 1996; Markland et al. 2000). The I50V mutation is located at the tips of the flaps (Fig. 1 ▶), which are characterized by a low degree of structural stability (Todd et al. 1998). In this paper, we report the binding thermodynamics of Amprenavir, one of six HIV-1 protease inhibitors approved for clinical use, and a chemically related second-generation inhibitor presently under development, TMC-126 (Yoshimura et al. 2002). TMC-126 has been shown to exhibit IC50s on the order of 0.3–0.5 nM against a wide spectrum of HIV, including common resistant mutations that severely affect existing protease inhibitors (Yoshimura et al. 2002). The chemical structures of the two inhibitors are shown in Figure 2 ▶. With this paper, we have concluded the thermodynamic characterization of five of the six protease inhibitors presently in clinical use against the same wild-type and drug-resistant mutations. The same characterization has also been performed on two second-generation inhibitors (KNI-764 and TMC-126) that show low susceptibility to drug-resistant mutations. The thermodynamic studies presented here permit us to draw general conclusions about the behavior of inhibitors and their response to mutations.
Fig. 1.
The structure of the HIV-1 protease indicating the location of the I50V (yellow) and V82F/I84V (red) inhibitor resistant mutations.
Fig. 2.
The chemical structures of Amprenavir and TMC-126.
Results
Binding energetics of Amprenavir to HIV-1 wild-type protease
The energetics of the association between the HIV-1 protease and the inhibitor Amprenavir was measured directly by isothermal titration calorimetry. Because Amprenavir is a tight-binding inhibitor of the HIV-1 protease, conventional titration experiments do not provide accurate estimates of the binding affinity, even though the binding enthalpy can be determined with high accuracy. To overcome this difficulty, calorimetric displacement titrations were performed, allowing for the determination of the affinity and enthalpy of binding at the same conditions as described before (Sigurskjold 2000; Velazquez-Campoy et al. 2001a). Figure 3 ▶ shows a typical calorimetric displacement titration for Amprenavir and wild-type HIV-1 protease. The left panel shows the direct titration of Amprenavir into the protease. This experiment indicates that Amprenavir binds to the wild-type HIV-1 protease with a favorable enthalpy change of −6.9 kcal/mole at 25°C. The experiment also indicates that the binding affinity is too high for accurate determination from a direct titration. Acetyl pepstatin was selected as the most appropriate weak inhibitor for displacement, because it shows a positive binding enthalpy that will amplify the magnitude of the signal in the displacement titration (Sigurskjold 2000; Velazquez-Campoy et al. 2001a). The center panel of Figure 3 ▶ shows the direct titration of acetyl pepstatin into the protease. The right panel shows the titration of Amprenavir into the HIV-1 protease prebound to acetyl pepstatin. From these experiments, we determined an association constant, Ka, for Amprenavir of 5.0 ± 0.3 × 109 M−1, corresponding to a Gibbs energy of binding of −13.2 kcal/mole. Accordingly, the binding of Amprenavir is almost equally favored by enthalpic (−6.9 kcal/mole) and entropic (−6.3 kcal/mole) contributions to binding. Additional experiments performed at different temperatures yield a heat capacity change of −440 ± 50 cal/K • mole, ΔCp = (∂ΔH/∂T)p, indicating that there is a significant hydrophobic desolvation effect upon binding. Other experiments performed in buffers with different enthalpies of ionization (acetate: ΔHion = 0.12 kcal/mole; MES: ΔHion = 3.72 kcal/mole; ACES: ΔHion = 7.51 kcal/mole; Fukada and Takahashi 1998) yielded similar binding enthalpies, indicating that under the conditions of the experiments (pH 5.0), there is no net proton transfer process coupled to inhibitor binding (Velazquez-Campoy et al. 2000). All the thermodynamic results of this paper are summarized in Table 1.
Fig. 3.
Calorimetric titrations of wild-type HIV-1 protease with Amprenavir (left panel), acetyl pepstatin (center panel), and with Amprenavir in the presence of acetyl pepstatin (displacement titration; right panel). These experiments were performed at 25°C in 10 mM acetate, pH 5, 2% DMSO. In all experiments the protease was in the calorimeter reaction cell and the inhibitors in the injection syringe. The inhibitor was added in stepwise injections of 10 μL. For each experiment the reactant concentrations were: (left panel) 23.7 μM protease, 250 μM Amprenavir; (center panel) 18.9 μM protease, 300 μM acetyl pepstatin; (right panel) 19.1 μM protease, 300 μM acetyl pepstatin, 250 μM Amprenavir.
Table 1.
Binding thermodynamics of TMC-126 and Amprenavir to HIV-1 protease wild-type and drug-resistant mutants I50V and V82F/I84V
| Protease | ΔG (kcal/mole) | −TΔS (kcal/mole) | ΔH (kcal/mole) | Ka (M−1) | Kd | Kd ratio | ΔCp (cal K−1 mole−1) | nH |
| TMC–126 | ||||||||
| Wild-type | −15.6 | −3.6 | −12.0 | (2.6 ± 0.1) × 1011 | (3.9 ± 0.1) pM | 1 | −350 ± 70 | 0.39 ± 0.02 |
| I50V | −13.9 | −4.8 | −9.1 | (1.6 ± 0.1) × 1010 | (61 ± 3) pM | 16 | −390 ± 30 | 0.16 ± 0.01 |
| V82F/I84V | −14.1 | −4.5 | −9.6 | (2.3 ± 0.1) × 1010 | (44 ± 2) pM | 11 | −460 ± 80 | 0.29 ± <0.01 |
| Amprenavir | ||||||||
| Wild-type | −13.2 | −6.3 | −6.9 | (5.0 ± 0.3) × 109 | (200 ± 11) pM | 1 | −440 ± 50 | 0.02 ± 0.03 |
| I50V | −10.3 | −6.1 | −4.2 | (3.4 ± 0.2) × 107 | (30 ± 2) nM | 147 | −400 ± 30 | 0.13 ± 0.03 |
| V82F/I84V | −10.5 | −6.6 | −3.9 | (4.8 ± 0.5) × 107 | (21 ± 2) nM | 104 | −370 ± 40 | −0.03 ± <0.01 |
The experiments were performed at 25°C, 10 mM sodium acetate, pH 5, 2% DMSO. Binding heat capacities and proton transfer numbers were determined by performing experiments at different temperatures and several buffers with different ionization enthalpies, respectively (see text for details).
Binding energetics of Amprenavir to HIV-1 mutant protease I50V
The binding of Amprenavir to the I50V mutant is also exothermic. However, the binding enthalpy to the mutant protein is 2.7 kcal/mole less favorable than to the wild-type protease. The binding affinity to the I50V mutant is reduced by a factor of 147 (3.4 × 107 M−1 or 30 nM), corresponding to a reduction in Gibbs energy of binding of 2.9 kcal/mole. Accordingly, the loss of binding affinity is almost entirely attributable to the less favorable enthalpy (ΔΔH = 2.7 kcal/mole) against the mutant protease. The heat capacity change upon binding was estimated to be −400 ± 30 cal/K • mole, similar to that obtained with the wild-type protease, consistent with a similar degree of desolvation. Again, experiments using buffers with different ionization enthalpies indicated no proton transfer upon binding. Together, the thermodynamic data are consistent with a weaker inhibitor/protein interaction, reflected only in the binding enthalpy.
Binding energetics of Amprenavir to HIV-1 mutant protease V82F/I84V
The binding of Amprenavir to the double-mutant protease is also exothermic; however, the binding enthalpy to the V82F/I84V mutant is ∼3 kcal/mole less favorable than to the wild-type protease. The double mutation reduces the binding affinity by a factor of 104, corresponding to a loss in Gibbs energy of binding of 2.7 kcal/mole, which can be almost completely accounted for by the loss of enthalpic interactions. The heat capacity change upon binding was estimated to be −370 ± 40 cal/K • mole, slightly smaller than that of the wild-type protease. As in the case of the I50V mutant, no net proton transfer process was observed upon binding.
Binding energetics of TMC-126 to HIV-1 wild-type protease
The binding energetics of the inhibitor TMC-126 to the HIV-1 protease was also measured by isothermal titration calorimetry (Fig. 4 ▶). TMC-126 binds very tightly to the protease with a very large favorable enthalpy change, −12.0 kcal/mole at 25°C (Fig. 4 ▶, left panel). From the displacement experiments an association constant, Ka, of 2.6 ± 0.1 × 1011 M−1 (3.9 ± 0.1 pM) was determined, corresponding to a Gibbs energy of binding of −15.6 kcal/mole. The calorimetric results indicate that for TMC-126 most of the binding energy is of enthalpic (−12.0 kcal/mole) rather than entropic (−3.6 kcal/mole) origin. For Amprenavir, on the other hand, the enthalpic and entropic contributions to the Gibbs energy of binding are similar. These results should be compared to those obtained for first-generation inhibitors (Indinavir, Nelfinavir, Saquinavir, Ritonavir), which showed entropically controlled binding affinities and unfavorable or only slightly favorable binding enthalpies (Todd et al. 2000). For these inhibitors, entropy contributions as large as −16 kcal/mole were required to compensate unfavorable binding enthalpies.
Fig. 4.
Calorimetric titrations of wild-type HIV-1 protease with the inhibitor TMC-126 (left panel), the inhibitor acetyl pepstatin (center), and with TMC-126 in the presence of acetyl pepstatin (displacement titration; right panel). These experiments were performed at 25°C in 10 mM acetate, pH 5, 2% DMSO. In all experiments the protease was in the calorimeter reaction cell and the inhibitors in the injection syringe. The inhibitor was added in stepwise injections of 10 μL. For each experiment the reactant concentrations were: (left panel) 6.4 μM protease, 84 μM TMC-126; (center panel) 18.9 μM protease, 300 μM acetyl pepstatin; (right panel) 6.6 μM protease, 400 μM acetyl pepstatin, 53 μM TMC-126.
The favorable binding enthalpy of TMC-126 is the largest that we have measured in our studies. KNI-764, another second-generation inhibitor, binds to the protease with a binding enthalpy of −7.6 kcal/mole (Velazquez-Campoy et al. 2001a). The origin of the favorable binding enthalpy of the KNI family of inhibitors and other low-molecular-weight hydrophobic molecules has been traced to the presence of bound water associated with the inhibitor (Leavitt and Freire 2001; Velazquez-Campoy et al. 2001a,b). Because the hydrophobicities of Amprenavir, KNI-764, and TMC-126 are rather similar, the large favorable enthalpy change observed for TMC-126 appears to be related to a larger number of water molecules trapped at the inhibitor/protein interface. In particular, TMC-126 seems to sequester a larger number of water molecules than the structurally related inhibitor Amprenavir and to establish stronger polar interactions with the catalytic region of the active site (Yoshimura et al. 2002). The additional number of structured water molecules together with the mostly polar interactions with Asp 29 and Asp 30 (Yoshimura et al. 2002) seem to account for the very favorable binding enthalpy of TMC-126.
Additional experiments performed at different temperatures yield a heat capacity change of −350 ± 70 cal/K • mole. Measurements performed in buffers with different enthalpies of ionization indicate that there is a net proton transfer (from the bulk solution to the protease–inhibitor complex) coupled to inhibitor binding. At pH 5.0, 0.39 ± 0.02 protons associate to the complex. Because TMC-126 does not have an ionizable group, the group responsible for the proton-transfer reaction must belong to the protease. Considering the structural similarity between Amprenavir and TMC-126, one could expect a similar net proton transfer for both inhibitors. However, the interactions of these two inhibitors with Asp 29 and Asp 30 are not the same. The bis-THF group in TMC-126 interacts very strongly with the backbone of the active-site amino acids Asp 29 and Asp 30 (Yoshimura et al. 2002). In particular, the two oxygens in the bis-THF group, which are absent in Amprenavir, are within hydrogen-bond distance of the main-chain amides of Asp 29 and Asp 30. This interaction may lead to a higher pKa increase in either of the carboxylate groups than the one observed with Amprenavir, and consequently a net proton absorption upon inhibitor binding.
Binding energetics of TMC-126 to HIV-1 mutant proteases
The binding energetics of TMC-126 to the resistant mutants I50V and V82F/I84V were also measured by isothermal titration calorimetry under identical conditions. The results are summarized in Table 1. The binding of TMC-126 to both mutants is also exothermic; however, the magnitudes of the binding enthalpies are 2.9 and 2.4 kcal/mole less favorable than that to the wild-type protease. The binding constants of TMC-126 to the resistant mutants are 1.6 × 1010 M−1 for I50V and 2.3 × 1010 M−1 for V82F/I84V, respectively. The I50V and V82F/I84V mutants lower the binding affinity for TMC-126 by a factor of 16 and 11, respectively, corresponding to a loss of 1.7 and 1.5 kcal/mole in Gibbs energy of binding. Because the loss in Gibbs energy is smaller than the loss in binding enthalpy, the results indicate the presence of compensatory entropic effects (−1.2 and −0.9 kcal/mole, respectively). Previously, Xie et al. (2000) indirectly estimated binding affinities of TMC-126 from the shift in protease stability in urea denaturation experiments. Although the absolute values differ from binding affinities measured directly (perhaps owing to different experimental conditions or the assumptions involved in the indirect method), the differences in binding affinities between wild-type and drug-resistant mutant V82F/I84V proteases are similar (1.5 and 1.3 kcal/mole).
The heat capacity changes upon binding were estimated to be −390 ± 30 and −460 ± 80 cal/K • mole for the I50V and V82F/I84V mutants, respectively, which appear to be slightly larger than the value measured for the wild-type protease. This observation would be consistent with a slightly better burial of the inhibitor with the mutant proteins. If this is the case, the compensatory entropic effect would originate from a higher desolvation of the inhibitor/mutant complexes. Similarly, the proton intakes measured for the inhibitor complexes with the mutant proteases are slightly smaller than the one measured for the wild-type protease.
The I50V mutation affects Amprenavir in a rather unique and severe fashion. Additional calorimetric experiments performed with existing clinical inhibitors (data not shown) reveal that the binding affinities of Indinavir, Nelfinavir, Saquinavir, and Ritonavir are only affected by a factor of 25, 55, 59, and 69 by the I50V mutation, respectively, compared with a factor of 147 for Amprenavir. The origin of the large effect of I50V on Amprenavir has been attributed to the strong interactions made by this inhibitor with the tips of the flap. The larger volume of the mutant cavity diminishes the packing density of the inhibitor and consequently the strength of the binding energy. Despite its structural similarity to Amprenavir, TMC-126 is not affected as much by the I50V mutation because most of its binding interactions are directed toward the bottom of the binding site, especially against Asp 29 and Asp 30 (Yoshimura et al. 2002).
Discussion
HIV-1 protease inhibitors are among the most powerful drugs available for the treatment of AIDS. However, the effectiveness of protease inhibitors is constantly threatened by the appearance of drug-resistant mutations in the protease molecule. Because viable protease mutations need to maintain catalytic activity and to be able to bind their substrates, they cannot occur randomly within the protease molecule. Knowing the location of conserved residues or residues that have a low mutation probability permits a more efficient targeting of binding interactions (Velazquez-Campoy and Freire 2001; Freire 2002). Understanding the nature and the limits of drug-resistant mutations provides the rationale for the development of protease inhibitors that are effective against existing inhibitor-resistant mutations and less susceptible to potential new mutations.
With the results presented in this paper, we have completed the thermodynamic characterization of five of the six protease inhibitors presently in clinical use and of two second-generation inhibitors. The experiments have been performed under identical conditions and against the same wild-type and drug-resistant mutations (Todd et al. 2000; Velazquez-Campoy et al. 2001a). Figure 5 ▶ summarizes the binding thermodynamics of all inhibitors measured so far. It is apparent from the data that second-generation inhibitors have significantly higher binding affinities than first-generation inhibitors, and that their binding affinity is determined by favorable enthalpy and entropy changes. The binding affinity of first-generation protease inhibitors is entropically driven and, except for Ritonavir, characterized by an unfavorable binding enthalpy.
Fig. 5.
Dissection of the binding energetics (ΔG = ΔH − TΔS) of five HIV-1 protease inhibitors presently in clinical use (Indinavir, Nelfinavir, Saquinavir, Ritonavir, Amprenavir) and two second-generation inhibitors (KNI-764 and TMC-126) to wild-type HIV-1 protease. In this figure, (solid bars) ΔG; (hatched bars) −TΔS; (cross-hatched bars) ΔH. The data for Indinavir, Nelfinavir, Saquinavir, and Ritonavir were reported in Todd et al. (2000), and the data for KNI-764 in Velazquez-Campoy et al. (2001a).
It is apparent from the results presented here that two characteristics are required for an inhibitor to maintain effectiveness against resistant mutations. First, an extremely high affinity against the main target (i.e., the wild-type protease); and, second, a mild response to mutations that permits the inhibitor to maintain a viable binding affinity and inhibitory effectiveness (Velazquez-Campoy and Freire 2001; Velazquez-Campoy et al. 2001a). In general, inhibitors lose a similar amount of binding enthalpy when confronted by a protease mutation. For example, TMC-126 loses 2.4 kcal/mole when confronted by the V82F/I84V mutation, which is similar to that experienced by Ritonavir. However, the binding affinity of TMC-126 decreases only by a factor of 11, whereas that of Ritonavir decreases by a factor of 370. A similar situation occurs with KNI-764, another second-generation inhibitor. Second-generation protease inhibitors like KNI-764 or TMC-126 appear to be able to compensate the binding enthalpy loss with an entropy gain as shown in Figure 6 ▶. The entropy gain is due to an increase in the number of degrees of freedom, either as a result of increased conformational dynamics within the complex or the release of water molecules associated with an increased desolvation. Either situation can be traced back to the ability of the inhibitor molecule to adapt to the binding-site distortions induced by the mutation. This is illustrated schematically in Figure 7 ▶, in which the binding-site cavities of the wild-type and V82F/I84V mutant protease are shown. In this figure the bulge and distortion created by the presence of the Phe ring are apparent. The total loss of two methyl groups in the I50V mutation (one per monomer) also changes the volume and geometry of the binding cavity without a change in polarity or hydrophobicity. These changes induce a loss of van der Waals interactions in all inhibitors that have been optimized against the wild type. This effect accounts for a similar decrease in the binding enthalpy for all inhibitors. However, conformationally constrained inhibitors respond differently than inhibitors showing a certain degree of flexibility or asymmetric functional groups in those regions facing the protein mutations. The loss of conformational constraints imposed by the elimination of certain interactions with the target allows a flexible inhibitor to search for a new energy minimum. An asymmetric functional group further permits an inhibitor to present different interaction surfaces against the mutated regions of the protein. A common situation will be one in which the inhibitor increases its burial from the solvent, thus increasing its desolvation and eliciting a more favorable binding entropy. A rigid or conformationally constrained inhibitor will not be able to trigger this effect. The experimental thermodynamic data support this hypothesis. It must be noted that an entropic compensation of this kind does not require an inhibitor to interact with a mutant as well as with the wild type. All that is required is that the lost interactions are compensated by an entropic gain.
Fig. 6.
Thermodynamic dissection of the effects of the inhibitor-resistant mutation V82F/I84V on the binding affinity of five HIV-1 protease inhibitors currently in clinical use (Indinavir, Nelfinavir, Saquinavir, Ritonavir, Amprenavir) and two second-generation inhibitors KNI-764 and TMC-126. In this figure, solid bars, ΔΔG; hatched bars, −TΔΔS; cross-hatched bars, ΔΔH. ΔΔX values are in reference to values obtained with the wild-type HIV-1 protease. The data for Indinavir, Nelfinavir, Saquinavir, and Ritonavir were reported in Todd et al. (2000), and the data for KNI-764 in Velazquez-Campoy et al. (2001a).
Fig. 7.
The binding-site cavity of the wild-type (left) and V82F/I84V (right) drug-resistant mutation. Indicated by a yellow arrow is the region where the bulge created by the phenylalanine ring at the edge of the cavity is located. The V82F/I84V mutation as well as the I50V mutation do not change the polarity or hydrophobic character of the binding site as indicated by the color scheme: (white) nonpolar, (green) polar, (blue) positive charge, (red) negative charge. These mutations only affect the geometry of the binding site. The figure was made with the program VMD (Humphrey et al. 1996).
Two properties appear to be important for lowering the susceptibility of an inhibitor to potential mutations. First, the strongest interactions must be directed against highly conserved residues with a very low probability to mutate. These residues can be identified by mapping genomic and stability information into the structure of the protein (Velazquez-Campoy and Freire 2001; Freire 2002). Asp 29 and Asp 30 fall into this category (Velazquez-Campoy and Freire 2001; Freire 2002) and appear to confer on TMC-126 its viability against mutations that affect other inhibitors. Second, the response to a mutation benefits from the presence of flexible elements or asymmetrical functional groups in those regions of the inhibitor facing target residues that have a high probability to mutate.
Materials and methods
Proteases
The genes encoding HIV-1 protease pseudo-wild-type (with three autolytic sites protected, Q7K/L33I/L63I) were transferred to the pET24 vector (Novagen), where expression is under the control of the T7 promoter. Mutations at selected positions (I50V and V82F/I84V) were introduced using an in vitro site-directed mutagenesis kit (QuikChange, Stratagene), and mutations were confirmed by DNA sequencing. Proteases were expressed in BL21/DE3 cells by adding IPTG to 1 mM once culture density (as determined by absorbance at 600 nm) was 1.5 or greater.
Protease purification
Plasmid-encoded HIV-1 protease was expressed as inclusion bodies in Escherichia coli 1458 (Todd et al. 1998, 2000; Todd and Freire 1999). Cells were suspended in extraction buffer (20 mM Tris, 1 mM EDTA, 10 mM 2-ME at pH 7.5) and broken with two passes through a French pressure cell (≥16,000 p.s.i.). Cell debris and protease-containing inclusion bodies were collected by centrifugation (20,000g at 4°C for 20 min). Inclusion bodies were washed with three buffers. Each wash consisted of resuspension (glass homogenizer, sonication) and centrifugation (20,000g at 4°C for 20 min). In each step a different washing buffer was used: Buffer-1 (25 mM Tris, 2.5 mM EDTA, 0.5 M NaCl, 1 mM Gly-Gly, 50 mM 2-ME at pH 7.0), Buffer-2 (25 mM Tris, 2.5 mM EDTA, 0.5 M NaCl, 1 mM Gly-Gly, 50 mM 2-ME, 1 M Urea at pH 7.0), Buffer-3 (25 mM Tris, 1 mM EDTA, 1 mM Gly-Gly, 50 mM 2-ME at pH 7.0). Protease was solubilized in 25 mM Tris, 1 mM EDTA, 5 mM NaCl, 1 mM Gly-Gly, 50 mM 2-ME, 9 M urea, pH 9.0, clarified by centrifugation, and applied directly to an anion exchange Q-Sepharose column (Q-Sepharose HP, Pharmacia) previously equilibrated with the same buffer. The protease was passed through the column and then acidified by adding formic acid to 25 mM immediately upon elution from the column. Precipitation of a significant amount of contaminants occurred upon acidification. Protease-containing fractions were pooled, concentrated, and stored at 4°C at 5–10 mg/mL.
The HIV-1 protease was folded by 10-fold stepwise dilution into 10 mM formic acid at 0°C. The pH was gradually increased to 3.8, then the temperature was raised to 30°C. Sodium acetate at pH 5.0 was added up to 100 mM, and protein was concentrated. Folded protease was desalted into 1 mM sodium acetate at pH 5.0 using a gel filtration column (PD-10, Pharmacia) and stored at either 4°C or −20°C (≥2.5 mg/mL) without loss of activity in several weeks. After folding, the protease was estimated to be ≥99% pure.
Determination of kinetic parameters
The catalytic activities of the HIV-1 proteases were tested prior to thermodynamic analysis by following the hydrolysis of the chromogenic substrate Lys-Ala-Arg-Val-Nle-nPhe-Glu-Ala-Nle-NH2, where Nle stands for norleucine and nPhe stands for p-nitrophenylalanine (California Peptide Research, Inc.).
In the spectrophotometric assay, protease was added to a 120-μL microcuvette containing substrate at 25°C. Final concentrations in the standard assay were: 30 nM to 60 nM active protease, 0 μM to 170 μM substrate, 10 mM sodium acetate, and 1 M sodium chloride, pH 5.0. The absorbance was monitored at six wavelengths (296–304 nm) using an HP 8452 diode array spectrophotometer (Hewlett Packard) and corrected for spectrophotometer drift by subtracting the average absorbance at 446–454 nm. An extinction coefficient for the difference in absorbance upon hydrolysis (1800 M−1 cm−1 at 300 nm) was used to convert absorbance change to reaction rates. Hydrolysis rates were obtained from the initial portion of the data, where at least 80% of the substrate remains free. The concentration of active protease was determined by performing active-site titrations with KNI-272, a very potent inhibitor (at pH 5.0, Ki ∼ 16 pM), using protease concentrations much higher (∼2 μM) than the corresponding Ki. The wild-type protease used in this work was characterized by kcat = 8.1 ± 0.2 sec−1, Km = 14 ± 1 μM; the V82F/I84V mutant by kcat = 6.4 ± 0.1 sec−1, Km = 28 ± 1 μM; and the I50V mutant by kcat = 6.4 ± 0.2 sec−1 and Km = 83 ± 2 μM under the same conditions.
Isothermal titration calorimetry
Isothermal titration calorimetry experiments were performed using a high-precision VP-ITC titration calorimetric system (Microcal Inc.). The enzyme solution in the calorimetric cell was titrated with Amprenavir, TMC-126, or acetyl pepstatin (Bachem AG) dissolved in the same buffer. The inhibitor concentration was estimated by nitrogen content determination (acetyl pepstatin) or from stoichiometric determination with a standardized protease solution. The heat evolved after each inhibitor injection was obtained from the integral of the calorimetric signal. The heat from the binding reaction between the inhibitor and enzyme was obtained as the difference between the heat of reaction and the corresponding heat of dilution. The binding affinities of Amprenavir and TMC-126 were determined by using ITC displacement experiments. Acetyl pepstatin was selected as the weak inhibitor in the displacement titrations because this inhibitor is endothermic and amplifies the signal of a high-affinity exothermic inhibitor when displaced. The measured binding enthalpies and binding affinities of acetyl pepstatin for the different HIV-1 proteases used in this paper were: wild-type: ΔH = 8.0 kcal/mole, Ka = 2.3 × 106 M−1; I50V mutant: ΔH = 11.1 kcal/mole, Ka = 6.9 × 104 M−1; V82F/I84V mutant: ΔH = 5.2 kcal/mole, Ka = 1.3 × 107 M−1. The extension and contribution of protonation/deprotonation processes to the binding was assessed by measuring the binding enthalpy in buffers with different enthalpies of ionization, that is, acetate, MES, and ACES as described before (Velazquez-Campoy et al. 2000). Analysis of the data was performed using software developed in this laboratory.
Acknowledgments
We thank J.W. Erickson for many helpful discussions; Tibotec for providing samples of TMC-126 and Amprenavir; and P. Wigerinck (Tibotec) for helpful comments on the manuscript. This work was supported by Grant GM 57144 from the National Institutes of Health.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
HIV, human immunodeficiency virus
IPTG, isopropyl-β-D-thiogalactoside
MES, 2-(N-morpholino)ethanesulfonic acid
ACES, N-(2-acetamido)-2-aminoethanesulfonic acid
EDTA, (ethylenedinitrile)tetraacetic acid
DMSO, dimethyl sulfoxide
ME, 2-mercaptoethanol
Tris, tris(hydroxymethyl)aminoethane
Gly-Gly, glycylglycine
ITC, isothermal titration calorimetry
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0206402.
References
- Ala, P.J., Huston, E.E., Klabe, R.M., McCabe, D.D., Duke, J.L., Rizzo, C.J., Korant, B.D., DeLoskey, R.D., Lam, P.Y.S., Hodge, C.N., and Chang, C.H. 1997. Molecular basis of HIV-1 protease drug resistance: Structural analysis of mutant proteases complexed with cyclic urea inhibitors. Biochemistry 36 1573–1580. [DOI] [PubMed] [Google Scholar]
- Ala, P.J., Huston, E.E., Klabe, R.M., Jadhav, P.K., Lam, P.Y.S., and Chang, C.-H. 1998. Counteracting HIV-1 protease drug resistance: Structural analysis of mutant proteases complexed with XV638 and SD146, cyclic urea amides with broad specificities. Biochemistry 37 15042–15049. [DOI] [PubMed] [Google Scholar]
- Condra, J.H., Schleif, W.A., Blahy, O.M., Gabryelski, L.J., Graham, D.J., Quintero, J.C., Rhodes, A., Robbins, H.L., Roth, E., Shivaprakash, M., et al. 1995. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature 374 569–571. [DOI] [PubMed] [Google Scholar]
- Freire, E. 2002. Designing drugs against heterogeneous targets. Nat. Biotech. 20 15–16. [DOI] [PubMed] [Google Scholar]
- Fukada, H. and Takahashi, K. 1998. Enthalpy and heat capacity changes for the proton dissociation of various buffer components in 0.1M potassium chloride. Proteins 33 159–166. [PubMed] [Google Scholar]
- Ho, D.D., Toyoshima, T., Mo, H., Kempf, D.J., Norbeck, D., Chen, C., Wideburg, N.E., Burt, S.K., Erickson, J.W., and Singh, M.K. 1994. Characterization of human immunodeficiency virus type 1 variants with increased resistance to a C2-symmetric protease inhibitor. J. Virol. 68 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, L., Treharne, A., Hartsuck, J.A., Foundling, S., and Tang, J. 1996. Crystal structures of complexes of a peptidic inhibitor with wild-type and two mutant HIV-1 proteases. Biochemistry 35 10627–10633. [DOI] [PubMed] [Google Scholar]
- Humphrey, W., Dalke, A., and Schulten, K. 1996. VMD—Visual molecular dynamics. J. Molec. Graph. 14 33–38. [DOI] [PubMed] [Google Scholar]
- Jadhav, P.K., Ala, P., Woerner, F.J., Chang, C.H., and Garber, S.S. 1997. Cyclic urea amides: HIV-1 protease inhibitors with low nanomolar potency against both wild type and protease inhibitor resistant mutants of HIV. J. Med. Chem. 40 181–191. [DOI] [PubMed] [Google Scholar]
- Kaplan, A.H., Michael, S.F., Wehbie, R.S., Knigge, M.F., Paul, D.A., Everitt, L., Kempf, D.J., Norbeck, D.W., and Erickson, J.W. 1994. Selection of multiple human immunodeficiency virus type 1 variants that encode viral proteases with decreased sensitivity to an inhibitor of the viral protease. Proc. Natl. Acad. Sci. 91 5597–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E.E., Baker, C.T., Dwyer, M.D., Murcko, M.A., Rao, B.G., R. D. Tung, R.D., and Navia, M.A. 1995. Crystal structure of HIV-1 protease in complex with VX_478, a potent and orally bioavailable inhibitor of the enzyme. J. Am. Chem. Soc. 117 1181–1182. [Google Scholar]
- Klabe, R.M., Bacheler, L.T., Ala, P.J., Erickson-Viitanen, S., and Meek, J.L. 1998. Resistance to HIV protease inhibitors: A comparison of enzyme inhibition and antiviral potency. Biochemistry 37 8735–8742. [DOI] [PubMed] [Google Scholar]
- Leavitt, S. and Freire, E. 2001. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 11 560–566. [DOI] [PubMed] [Google Scholar]
- Markland, W., Rao, B.G., Parsons, J.D., Black, J., Zuchowski, L., Tisdale, M., and Tung, R. 2000. Structural and kinetic analyses of the protease from an Amprenavir-resistant human immunodeficiency virus type 1 mutant rendered resistant to Saquinavir and resensitized to Amprenavir. J. Virology 74 7636–7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz, M., Mo, H., Kempf, D.J., Noebeck, D.W., Bhat, T.N., Erickson, J.W., and Ho, D.D. 1995. Selection and analysis of human immunodeficiency virus type 1 variants with increased resistance to ABT-538, a novel protease inhibitor. J. Virol 69 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazhanisamy, S., Stuver, C.M., Cullinan, A.B., Margolin, N., Rao, B.G., and Livingston, D.J. 1996. Kinetic characterization of human immunodeficiency virus type-1 protease-resistant variants. J. Biol. Chem. 271 17979–17985. [DOI] [PubMed] [Google Scholar]
- Roberts, N.A. 1995. Drug-resistance patterns of Saquinavir and other HIV proteinase inhibitors. AIDS 9 S27–S32. [PubMed] [Google Scholar]
- Sigurskjold, B.W. 2000. Exact analysis of competition ligand binding by displacement isothermal titration calorimetry. Anal. Biochem. 277 260–266. [DOI] [PubMed] [Google Scholar]
- Tisdale, M. 1996. HIV protease inhibitors—Resistance issues. Int. Antiviral News 4: 95–107. [Google Scholar]
- Todd, M.J. and Freire, E. 1999. The effect of inhibitor binding on the structural stability and cooperativity of the HIV-1 protease. Proteins 36 147–156. [DOI] [PubMed] [Google Scholar]
- Todd, M.J., Semo, N., and Freire, E. 1998. The structural stability of the HIV-1 protease. J. Mol. Biol. 283 475–488. [DOI] [PubMed] [Google Scholar]
- Todd, M.J., Luque, I., Velazquez-Campoy, A., and Freire, E. 2000. The thermodynamic basis of resistance to HIV-1 protease inhibition. Calorimetric analysis of the V82F/I84V active site resistant mutant. Biochemistry 39 11876–11883. [DOI] [PubMed] [Google Scholar]
- Velazquez-Campoy, A. and Freire, E. 2001. Incorporating target heterogeneity in drug design. J. Cell. Biochem. S37 82–88. [DOI] [PubMed] [Google Scholar]
- Velazquez-Campoy, A., Luque, I., Todd, M.J., Milutinovich, M., Kiso, Y., and Freire, E. 2000. Thermodynamic dissection of the binding energetics of KNI-272, a powerful HIV-1 protease inhibitor. Protein Sci. 9 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez-Campoy, A., Kiso, Y., and Freire, E. 2001a. The binding energetics of first and second generation HIV-1 protease inhibitors: Implications for drug design. Arch. Biochim. Biophys. 390 169–175. [DOI] [PubMed] [Google Scholar]
- Velazquez-Campoy, A., Luque, I., and Freire, E. 2001b. The application of thermodynamic methods in drug design. Thermochim. Acta 380 217–227. [Google Scholar]
- Xie, D., Gulnik, S., and Erickson, J.W. 2000. Dissection of binding energy with native and ligand-bound protein stabilities: Determining the affinity of ultratight-binding inhibitors of HIV-1 protease and its drug resistance mutants. J. Am. Chem. Soc. 122 11533–11534. [Google Scholar]
- Yoshimura, K., Kato, R., Kavlick, M.F., Nguyen, A., Maroun, V., Maeda, K., Hussain, K.A., Ghosh, A.K., Gulnik, S.V., Erickson, J.W., and Mitsuya, H. 2002. A potent human immunodeficiency virus type 1 protease inhibitor, UIC-94003 (TMC-126), and the selection of a novel (A28S) mutation in the protease active site. J. Virol. 76 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]