Abstract
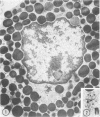
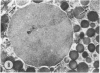
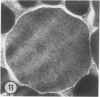
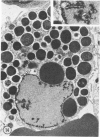
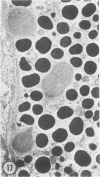
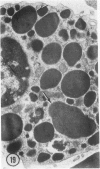
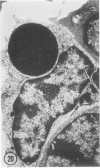
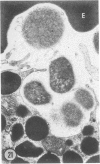
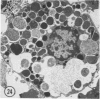
The prevalence of mast cells infiltrating bone marrow of different rats varied widely, as did the staining properties and size of their cytoplasmic granules. Bone marrow mast cells from several rats revealed large membrane-limited inclusions which stained metachromatically or orthochromatically and resembled inclusions in some macrophages. Ultrastructurally, mast cells varied widely in content of uniform dense granules or enlarged granules with less dense, fine grained content. Some of the large inclusions observed ultrastructurally in mast cells were heterophagic vacuoles which contained erythrocytes or reticulocytes, or remnants from other phagocytized cells, possibly neutrophils or unidentified homogeneous material. Smaller bodies, interpreted as fragments of erythrocytes, lay extracellularly near mast cells and occupied small, membrane-limited, heterophagic vacuoles in some mast cells. In other mast cells, communal vacuoles enclosed several specific cytoplasmic granules in various stages of disruption. The communal vacuoles occasionally opened to the extracellular space. A few large indeterminate vacuoles in mast cells contained amorphous flocculent matter which apparently derived either from coalescence of cytoplasmic granules through fusion of granule membranes or from endocytosis.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colvin R. B., Dvorak A. M., Dvorak H. F. Mast cells in the cortical tubular epithelium and interstitium in human renal disease. Hum Pathol. 1974 May;5(3):315–326. doi: 10.1016/s0046-8177(74)80114-0. [DOI] [PubMed] [Google Scholar]
- ESSNER E. An electron microscopic study of erythrophagocytosis. J Biophys Biochem Cytol. 1960 Apr;7:329–334. doi: 10.1083/jcb.7.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Reversed type allergic skin reactions by anti-gamma-E-globulin antibodies in humans and monkeys. J Immunol. 1968 Mar;100(3):554–562. [PubMed] [Google Scholar]
- Komiyama A., Spicer S. S. Acid phosphatase demonstrated ultrastructurally in mast cell granules altered by pinocytosis. Lab Invest. 1975 Apr;32(4):485–491. [PubMed] [Google Scholar]
- LAGUNOFF D., BENDITT E. P. Proteolytic enzymes of mast cells. Ann N Y Acad Sci. 1963 Feb 26;103:185–198. doi: 10.1111/j.1749-6632.1963.tb53698.x. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. Membrane fusion during mast cell secretion. J Cell Biol. 1973 Apr;57(1):252–259. doi: 10.1083/jcb.57.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney M. A., Patt H. M., Lund J. E. Granulocyte dynamics and the question of ineffective granulopoiesis. Cell Tissue Kinet. 1971 Mar;4(2):201–209. doi: 10.1111/j.1365-2184.1971.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Moriyasu S., Yamura T. Electron microscopic studies of mast cell degranulation. Acta Derm Venereol Suppl (Stockh) 1973;73:149–156. [PubMed] [Google Scholar]
- Padawer J. Ingestion of colloidal gold by mast cells. Proc Soc Exp Biol Med. 1968 Dec;129(3):905–907. doi: 10.3181/00379727-129-33455. [DOI] [PubMed] [Google Scholar]
- Padawer J. Phagocytosis of particulate substances by mast cells. Lab Invest. 1971 Oct;25(4):320–330. [PubMed] [Google Scholar]
- Parish W. E. Release of histamine and slow reacting substance with mast cell changes after challenge of human lung sensitized passively with reagin in vitro. Nature. 1967 Aug 12;215(5102):738–739. doi: 10.1038/215738a0. [DOI] [PubMed] [Google Scholar]
- SPICER S. S. Histochemical properties of mucopolysaccharixe and basic protein in mast cells. Ann N Y Acad Sci. 1963 Feb 26;103:322–333. doi: 10.1111/j.1749-6632.1963.tb53707.x. [DOI] [PubMed] [Google Scholar]
- Sheard P., Killingback P. G., Blair A. M. Antigen induced release of histamine and SRS-A from human lung passively sensitized with reaginic serum. Nature. 1967 Oct 21;216(5112):283–284. doi: 10.1038/216283a0. [DOI] [PubMed] [Google Scholar]
- Simson J. V., Spicer S. S. Ferritin particles in macrophages and in associated mast cells. J Cell Biol. 1972 Mar;52(3):536–541. doi: 10.1083/jcb.52.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer S. S. Siderosis Associated with Increased Lipofuscins and Mast Cells in Aging Mice. Am J Pathol. 1960 Oct;37(4):457–475. [PMC free article] [PubMed] [Google Scholar]
- Tasaka K., Yamasaki H. Local degranulation and histamine release from a single rat mast cell by microelectrophoretic application of basic histamine releasers and antigen. Acta Derm Venereol Suppl (Stockh) 1973;73:167–173. [PubMed] [Google Scholar]
- Uvnäs B. Histamine storage and release. Fed Proc. 1974 Oct;33(10):2172–2176. [PubMed] [Google Scholar]