Abstract
Predators and their odors offer an ethologically valid model to study learning processes. The present series of experiments assessed the ability of ferret odor to serve as an unconditioned stimulus and examined behavioral and endocrine changes in male Sprague–Dawley rats with single or repeated exposures in a defensive withdrawal paradigm or in their home cages. Rats exposed to ferret odor avoided the ferret odor stimulus more, exhibited greater risk assessment and displayed higher adrenocorticotropin hormone (ACTH) and corticosterone release compared with control odor exposed rats and these measures did not significantly habituate over repeated exposures. Ferret odor exposure did not show associative conditioning effects during extinction trials. However, rats that were pre-exposed to ferret odor only once, as compared to control and repeatedly exposed rats, displayed a sensitized ACTH and corticosterone response to an additional ferret odor exposure in small cages. These experiments suggest that ferret odor is a highly potent unconditioned stimulus that has long lasting effects on behavior and endocrine responses, and further suggests the independence of habituation and sensitization processes.
Keywords: ACTH, Conditioning, Corticosterone, Defensive withdrawal, Habituation, Ferret odor, Predator, Sensitization
1. Introduction
Fear of predation offers a unique model to study unconditioned fear. The presentation of predators or cues associated with predators to rodents elicit unconditioned fear responses, such as increases in the stress hormones, corticosterone and adrenocorticotropin hormone (ACTH), [1–6] and defensive behavioral responses [2–4,7–10]. Laboratory rats have an innate fear of predators even if they have never previously encountered a predator. This ethologically valid model provides a useful tool for the study of both non-associative and associative learning.
Habituation, which is considered a non-associative form of learning, is described as a decrease in the strength of a response to a stimulus after repeated presentations of that stimulus [11,12]. One general principle of habituation is that the more intense a stimulus is, the slower, if at all, the response will habituate [12]. A live predator, like a cat, would be a very intense fear stimulus to a rat because of potential harm or even death. Previous studies have shown that intense fear or defensive reactions of rats to live cats do not habituate over several exposures [2]. But studies examining repeated cat odor exposures to rats have provided mixed results. Odors of predators have been considered partial cues because they produce a less intense reaction than live predator exposure [10,13–16]. In some studies behavioral responses to cat odor do not habituate over several sessions [17], while in others some responses do habituate [18], or even increase after several exposures [19]. The inconsistencies of the cat odor studies may be due to the different exposure paradigms used or because cat odor in general produces variable stress responses depending on rat strain [19–21]. Ferret odor exposure over several sessions has not previously been examined and would provide an additional data set on habituation of responses to predator odors. Domestic ferrets (Mustela putorius furo) are natural predators of rodents [1,22,23]. Both live ferrets and ferret fur/skin odor increase plasma corticosterone and ACTH in rats in laboratory settings [1,4–6]. Behavioral avoidance of ferret fur/skin odor by rats in a defensive withdrawal paradigm has also been reported [4].
Conditioning is an associative form of learning. The subject learns to associate an unconditioned stimulus (such as an odor or loud noise) with another stimulus (such as a novel chamber or another stimulus) and after forming the association the conditioned stimulus will elicit some of the responses that the unconditioned stimulus elicited. The strength of the association formed will depend on several factors including the number of pairings of the unconditioned and neutral stimuli and prior familiarity with the neutral stimuli [24]. Live cat and cat odor have been previously shown to support conditioning [10,15,25,26]. The ability of ferret odor to support conditioning has not been previously examined and this information could further the utility of ferret odor in associative learning.
The present studies utilized a defensive withdrawal paradigm [16,27–29] to determine if ferret odor alone could serve as an unconditioned stimulus in an associative learning paradigm, and if repeated presentations of the odor would lead to habituation of the behavioral responses. Plasma ACTH and corticosterone responses to the ferret odor were also examined in an exposure paradigm after 10 days of the defensive withdrawal testing. In a separate experiment, defensive withdrawal behavior in response to ferret odor was tested without pre-exposure to the testing apparatus. This was done to determine the influence of prior experience on context or cue conditioning. A final experiment examined the endocrine (ACTH and corticosterone) response to repeated ferret odor exposure in the rats' home cages. Some of these data have been presented in abstract form [30].
2. Methods of Experiment 1
Experiment 1 examined the behavior of rats exposed to ferret odor once, repeatedly, or to a control odor in a defensive withdrawal apparatus. The design determined whether repeated ferret odor exposure would lead, or not, to behavioral habituation of the avoidance responses, and whether ferret odor could serve as an unconditioned stimulus in a conditioning paradigm. ACTH and corticosterone responses to ferret odor exposure in a small cage after 10 days of defensive withdrawal testing were also investigated.
2.1. Subjects
Twenty-six male Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 250–300 g were used. They were housed in a dedicated colony facility and grouped four to five in clear polycarbonate cages (48 × 27 × 20 cm) containing floor wood shavings, and covered with wire lids providing food (rat chow) and water ad libitum. Animals were housed for a period of at least 7 days after arrival from the supplier, before any experimental manipulations were conducted. They were kept on a controlled reverse light/dark cycle (lights off 7:00 am — on at 7:00 pm), under constant humidity and temperature conditions. All procedures were performed between 9:00 am and 12:00 pm to reduce variability due to normal circadian hormonal variations. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado and conformed to the United States of America National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Odor stimuli
Ferret odor was collected by placing a bath towel in a cage with 1 male and 1 female undescented adult ferrets for approximately 1 month (gift from Dr. V. Staton, Ohio Dominican Univ.). The towel was cut into 5 × 5 cm squares, sealed in a plastic bag, express mailed overnight and then placed in an −80 °C freezer until used. The control towels were new clean bath towels. The towels were transported to the experimental rooms immediately before testing inside sealed glass bell jars.
2.3. Behavior apparatus
The apparatus was a 58 × 58 × 39 cm Plexiglas open field chamber with a metal 29 × 20 × 14 cm chamber in one corner with an opening on the long side that was 9 cm wide and 8 cm tall. The floor and sides of the open field were black and white tape was used to delineate 16 equal sized squares on the floor, of which the smaller chamber occupies 2 full squares and half of two squares. The open field was elevated to a height of 53 cm. The room that contained the apparatus was dimly lit by a 75-watt light and white noise (60 dB Sound Pressure Level) was provided by an AM7 Grass Medical Instruments audio monitor (Quincy, MA). Behavior was videotaped (Sony VHS recorder) by a Panasonic WV-BP130 video camera (Ontario, Canada) mounted directly above the apparatus (approximately 2.4 meters) to the ceiling.
2.4. Procedure
Seven days after arrival from the supplier, rats were individually exposed to the experimental apparatus. Each rat was placed in the apparatus for 10 min on 4 consecutive days at approximately the same time each day. All behavioral testing was conducted during the rats' dark phase. The day after completion of acclimation, rats were divided into three groups: extinction group (n=9), habituation group (n=8), and control group (n=9). The extinction group was exposed to a towel with ferret odor on day 1 but then exposed to control towels on days 2–7 in the behavior apparatus. The habituation group was exposed to ferret towels in the behavior apparatus on days 1–7 and then control towels days 8–10. The control group was exposed to control towels on days 1–10. The towels were taped to the floor of the apparatus in the diagonal corner opposite the small metal chamber. The rat was then placed in the center of the apparatus and behavior was videotaped for 10 min. The apparatus was carefully cleaned with a 10% chlorine bleach solution between each rat to reduce olfactory cues. New pieces of towel were used for each subject and rats were run in the same order each day.
2.5. Video analysis
Two researchers blind to the experimental conditions analyzed the videotaped behavior. Behaviors analyzed include: number of entries to the withdrawal chamber, time spent in withdrawal chamber (seconds), number of visits to the corner containing the towel (front paws in towel square), scanning behavior (number of slow horizontal head movements), and immobility (complete immobility except for breathing). The scores of the two researchers were averaged. Scorers achieved a level of 95%–99% agreement depending on measure.
2.6. Odor presentation
On day 11, rats were placed individually in 28 × 18 × 14 cm plastic cages with wire lids and transported to remote experimental rooms (kept on the same light cycle) to avoid manipulation and transport of the rats immediately prior to odor exposure. Rats had access to food and water during the entire experiment. The next morning (day 12), 2 pieces of towel with ferret odor were carefully placed at each end of all the cages without disturbing the rats by hooking the towels to the wire cage lid with paper clips, so the towels hung inside the cage. Dividers were placed between each cage so that rats could not see each other, but vocal communication was still possible. Immediately following the 30 min exposure, rats were taken to an adjacent room, rapidly decapitated and trunk blood was collected. The same experimental room was used only once a day to ensure dissipation of any residual smell. Two rats (1 from conditioning group and 1 from control group) escaped when their cages were opened and therefore were not included in this analysis.
2.7. Corticosterone and ACTH radioimmunoassays
Blood was collected into ice-chilled tubes containing EDTA (20 mg/ml). Blood samples were centrifuged at 2000 rpm for 10 min, the plasma was pipetted into 0.5 ml Ependorf microcentrifuge tubes, and stored at −80 °C until assayed.
Corticosterone was measured by radioimmunoassay using a specific rabbit antibody (gift from Dr. S. Watson, Univ. Michigan), with less than 3% crossreactivity with other steroids. Plasma samples were diluted 1:100 in 0.05 M sodium phosphate buffer containing 0.25% bovine serum albumin (BSA) pH 7.4, and corticosterone separated from binding protein by heat (70 °C, 30 min). Duplicate samples of 200 μl to which 50 μl of trace (3H-corticosterone; Amersham 50 Ci/mmol, 10,000 cpm/tube) and 50 μl of antibody (final concentration 1:12,800) were incubated at 4 °C overnight. Separation of bound from free corticosterone was achieved by adding 0.5 ml of chilled 1% charcoal–0.1% dextran mixture in buffer for 10 min at 4 °C and centrifuged for 10 min at 3000 rpm (Eppendorf/Brinkman 5810R). The supernatant was poured into 5 ml scintillation fluid and bound 3H-corticosterone counted on a Packard Instruments (Model 1600TR) liquid scintillation analyzer and compared to a standard curve (range: 0–80 μg/dl). All samples from an experiment were measured simultaneously to reduce interassay variability; within assay variability between duplicates was less than 8%.
ACTH was measured with a kit (ACTH 130T kit — Cat. No. 40-2195) from Nichols Institute Diagnostics (San Clemente, CA) according to the manufacturer's protocol. The sensitivity of the assay ranged from 5 to 1400 pg/ml. All samples from an experiment were measured simultaneously to reduce interassay variability.
2.8. Data analysis
Day 1 behavioral data and day 12 ACTH and corticosterone data were analyzed using multivariate analyses of variance (MANOVA). Tukey's honestly significant difference (HSD) multiple means comparisons were used to analyze post hoc differences (p<.05). Day 2 behavioral data were also analyzed using MANOVA and Tukey's HSD test to determine if rapid conditioning occurred. Days 2–7 and days 8–10 data were analyzed using repeated measures MANOVAs (Pillai's Trace) with days and measures as the repeated measures and group as the between-subjects factor. Day 7 behavioral data were also analyzed using MANOVA and Tukey's HSD test to determine if habituation occurred.
3. Methods of Experiment 2
Experiment 2 was conducted to determine if conditioning of some of the defensive withdrawal behavior would emerge without prior exposure to the behavior apparatus, as was done in Experiment 1. Rats were exposed to ferret odor or control odor on day 1 of testing and exposed to control odor on day 2. This experiment would minimize the possibility that latent inhibition of the behavioral apparatus through prior pre-exposure precluded the measurement of conditioned behavior in Experiment 1 [31].
3.1. Subjects
Sixteen male Sprague–Dawley rats were used in this experiment. Rats were housed and fed as described in Experiment 1. The rats in this experiment were also on a reversed light–dark cycle (lights off 7:00 am — on at 7:00 pm). All procedures were performed between 9:00 am and 12:00 pm to reduce variability due to normal circadian hormonal variations.
3.2. Procedure
Seven days after arrival from the supplier, rats were individually tested in the defensive withdrawal paradigm for 2 days, as described in Experiment 1. On day 1, eight rats were exposed to ferret odor on towels and eight rats were exposed to control odor towels. On day 2, all the rats were exposed to control odor towels. The apparatus was carefully cleaned with a 10% chlorine bleach solution between each rat to reduce olfactory cues. New pieces of towel were used for each subject and rats were run in the same order each day. The rats' behavior was videotaped for 10 min and scored as previously described.
3.3. Data analysis
Behavioral data were analyzed on days 1 and 2 using multivariate ANOVA (Pillai's Trace, p<.05). Data from both days were also analyzed using repeated measures MANOVA with days and measures as the repeated measures and group as the between-subjects factor (p<0.05), to determine overall effects.
4. Methods of Experiment 3
Experiment 3 examined the endocrine effects of repeated ferret odor exposure in the rats' home cages. Plasma ACTH and corticosterone were analyzed on days 1, 4, and 9. This experiment was conducted to determine if endocrine changes seen after one exposure to ferret odor would habituate after repeated exposures.
4.1. Subjects
Sixteen male Sprague–Dawley rats were used in this experiment. Rats were housed and fed as described in Experiment 1.
4.2. Procedure
The evening prior to day 1 of testing, rats were single housed in 46 × 25 × 22 cm plastic cages with wire lids and divided into two groups: ferret odor exposed and control odor exposed. The groups were transported to separate experimental rooms (kept on the same light cycle) to avoid manipulation and transport of the rats immediately prior to odor exposure and to avoid odor contamination. Rats had access to food and water during the entire experiment. For 9 days, two pieces of towel with ferret odor or control odor were carefully placed at each end of the cages without disturbing the rats by hooking the towels to the wire cage lid with paper clips, so the towels hung inside the cage for 30 min. Dividers were placed between each cage so that rats could not see each other, but vocal communication was still possible. On days 1, 4, and 9, immediately following the 30 min exposure, rats were taken to an adjacent room and blood was taken via tail nicks. ACTH and corticosterone radioimmunoassays were conducted as described in Experiment 1.
4.3. Data analysis
Plasma ACTH and corticosterone levels were analyzed using repeated measures MANOVAs (Pillai's Trace) with days and endocrine hormones as the repeated measures and group as the between-subjects factor (p<0.05).
5. Results of Experiment 1
5.1. Behavior day 1
On day 1, the habituation and extinction groups were exposed to ferret odor on a towel and the control group was exposed to a new piece of towel (control towel) in the defensive withdrawal paradigm and behavior was analyzed. MANOVA revealed a significant group effect: F(8, 42)=2.85, p=.013 (Pillai's Trace). As Fig. 1 suggests, significant differences between groups were found for entries to withdrawal chamber: F(2, 25)=10.00, p=.001, visits to towel: F(2, 25)=5.14, p=.014, and head scanning movements, F(2, 25)=6.38, p=.006. Post hoc Tukey's HSD comparisons revealed that ferret odor exposed rats entered the withdrawal chamber and visited the towel less than control odor exposed rats and also exhibited more horizontal head scanning movements than control odor exposed rats. No significant differences were found between groups on day 1 for time spent in the withdrawal chamber and immobility.
Fig. 1.
Graphs showing mean (±SEM) behavior in defensive withdrawal apparatus for extinction (n=9), habituation (n=8), and control (n=9) groups for 10 min/day for 10 days. On day 1, both extinction and habituation groups were exposed to ferret odor towels. On days 2–7, only the habituation group was exposed to the ferret odor towels. And, days 8–10, habituation and control groups were exposed to control odor towels. Panel A depicts number of entries to the withdrawal chamber. Panel B depicts time spent (s) in withdrawal chamber. Panel C depicts number of visits by the rats to the towel stimulus. Panel D depicts number of head scanning movements. *Indicates a significant difference between control and habituation groups (p<0.05). #Indicates a significant difference between control and extinction groups (p<0.05).
5.2. Behavior day 2
On days 2–7, the habituation group was exposed to ferret odor on a towel each day and the control and extinction groups were exposed to a new piece of towel (control towel) each day in the defensive withdrawal paradigm. If rapid conditioning took place, the extinction group's day 2 behavior would look similar to the habituation group's behavior. If rapid conditioning did not take place, the extinction group's behavior would look similar to the control group's behavior. MANOVA revealed a significant group effect: F(8, 42)=3.41, p=.004. Differences between groups on day 2 were found for entries to chamber: F(2, 25)=6.49, p=.006, visits to towel: F(2, 25)=10.58, p=.001, and head scanning: F(2, 25)=4.94, p=.016. The extinction group's behavior was significantly different from the habituation group's behavior and not different from the control group's behavior, as shown in Fig. 1. The habituation group entered the chamber less and scanned more than the control group and the extinction group was not significantly different from the control group. The extinction group visited the towel more than the habituation group. The extinction group's behavior was also similar to the control group's behavior for visits to the towel and head scans. No significant differences between groups were found on day 2 for time spent in chamber and immobility.
5.3. Behavior days 2–7
To determine if habituation to the ferret towel occurred, the habituation group (that was exposed to ferret odor on all 7 days) was compared to the control group (that was exposed to the control odor), on days 2–7. If significant habituation took place, the habituation group's behavior should have become similar to the control group's behavior over repeated ferret odor presentations. A repeated measures MANOVA revealed significant days, measures, and group effects, and importantly measures × group, F(3, 13)=5.09, p=.015 and days × mea-measures, F(3, 13)=4.42, p=.024, effects, suggesting that the different measures quantified varied with odor presentation. MANOVA on day 7 behavior revealed a significant group effect: F(4, 12)=3.32, p=.048. Differences between groups on day 7 were found for time in chamber: F(1, 16)=9.97, p=.007, visits to towel: F(1, 16)=7.24, p=.017, and head scanning: F(1, 16)=6.69, p=.021. No significant differences between groups were found for entries to chamber and immobility. These data suggest that habituation to repeated ferret odor exposures did not occur to many of the behaviors quantified.
5.4. Behavior days 8–10
On days 8–10, the habituation and control groups were exposed to the control odor in the defensive withdrawal paradigm to determine if several previous exposures to ferret odor (habituation group) would lead to conditioning when this group was subjected to an extinction procedure (control odor). As observed in Fig. 1, no significant group differences between the habituation and control groups were found for any measure: entries to chamber, time in chamber, visits to towel, immobility, and scanning behavior.
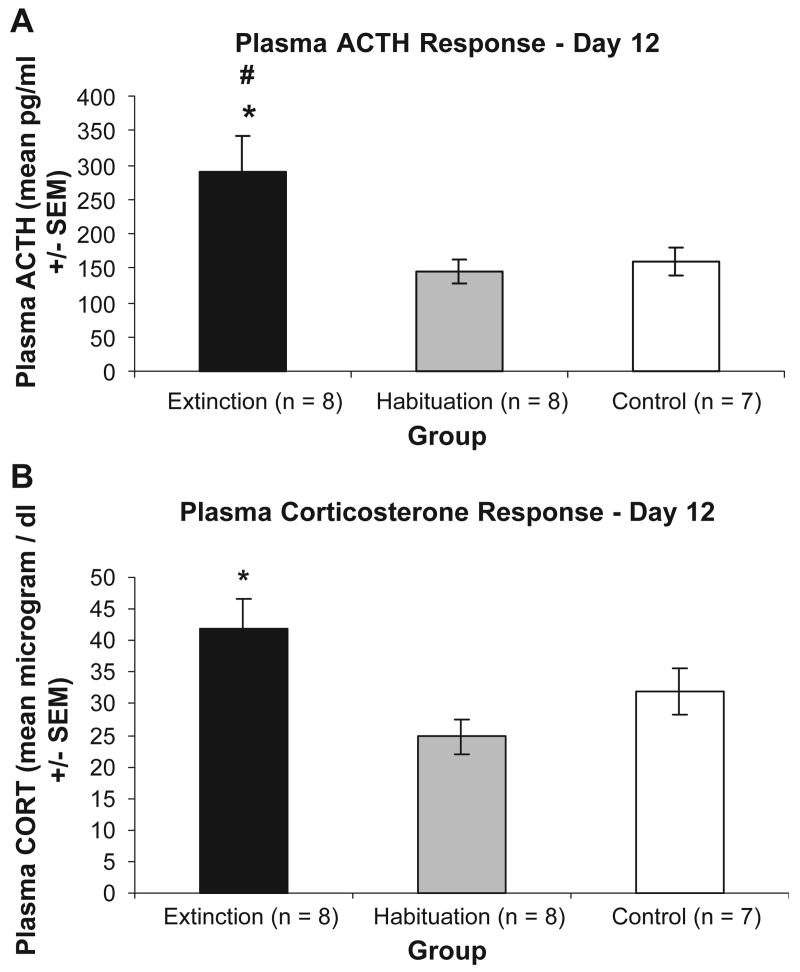
5.5. Day 12 ACTH and corticosterone
As shown in Fig. 2, a significant difference between groups was found for day 12 ACTH and corticosterone responses to ferret odor exposure, F(4, 40)=3.39, p=.018. Post hoc Tukey's HSD comparisons revealed significantly higher mean ACTH and corticosterone levels for the extinction group compared to the habituation group (p's=.008 and .009, respectively). ACTH levels for extinction rats were also significantly different from the control rats (p=.02), but CORT levels did not differ between these groups. ACTH and CORT levels for the habituation and control groups did not significantly differ.
Fig. 2.
Graphs showing mean (±SEM) plasma levels of ACTH (Panel A) and corticosterone (Panel B) for extinction (n=8), habituation (n=8) and control (n=7) group rats exposed to ferret odor for 30 min on day 12. *Indicates a significant difference between extinction and habituation groups (p<0.05). #Indicates a significant difference between control and extinction groups (p<0.05).
6. Results of Experiment 2
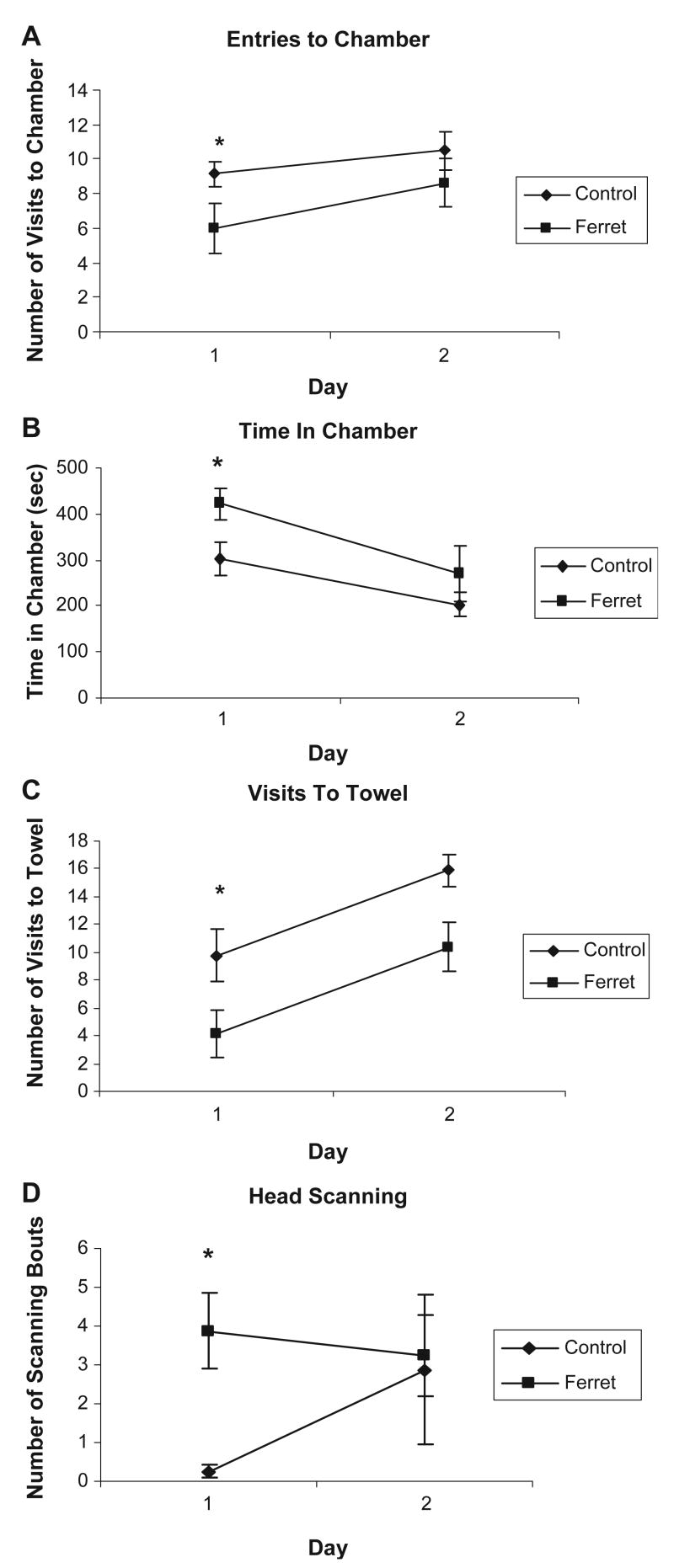
In Experiment 2, rats were tested in the defensive withdrawal apparatus for two days without pre-exposure to the apparatus. On day 1, rats were exposed to ferret odor towels or control towels in the apparatus. On day 2, all rats were exposed to control odor towels to determine if behavioral responses to the ferret towel persisted the second day without the ferret odor cue (conditioned). A repeated measures MANOVA on the two days revealed significant main days and measure effects and importantly, measure × group, F(3, 12)=5.38, p=.014, and days × measure, F(3, 12)=4.23, p=.030 effects without a main group effect. MANOVA on day 1 behavior revealed a significant group effect: F(4, 11)=4.13, p=.028. As shown in Fig. 3, significant group differences were found for time spent in chamber, F(1, 15)=5.64, p=.032, visits to towel, F(1, 15)=4.94, p=.043, and scanning, F(1, 15)=13.53, p=.002. MANOVA on day 2 revealed no significant group × measure effect indicating the lack of a clear conditioning effect of ferret odor.
Fig. 3.
Graphs showing mean (±SEM) behavior in defensive withdrawal apparatus for control odor group (n=8) and day 1 ferret odor-exposed group (n=8) for 10 min on days 1 and 2. Panel A depicts number of entries to the withdrawal chamber. Panel B depicts time spent (sec) in withdrawal chamber. Panel C depicts number of visits to the towel stimulus. Panel D depicts number of head scanning movements. *Indicates a significant difference between groups (p<0.05).
7. Results of Experiment 3
In Experiment 3, rats were exposed to ferret odor or control odor towels in individual home cages for 30 min/day for 9 days. Plasma ACTH and corticosterone responses were determined from blood taken on days 1, 4, and 9. A repeated measures MANOVA revealed significant main effects of days, measures, and groups (all p's<.002), various interactions, but importantly, a triple days × measures × group, F(2, 13)=4.55, p=.032, interaction effect. This effect indicates differential responsiveness of the hormonal indices in the groups receiving ferret or control odors over days. This was further revealed by MANOVAs with significant group effects for corticosterone on day 1, F(1, 15)=6.75, p=.021, but not days 4 or 9, and ACTH on days 1 and 4 (p's<.02), but not 9, as shown in Fig. 4.
Fig. 4.
Graphs showing mean (±SEM) plasma levels of ACTH (Panel A) and corticosterone (Panel B) for control (n=8) and ferret odor exposed rats (n=8) on days 1, 4, and 9 of repeated home cage exposure to odors. *Indicates a significant difference between control and ferret odor groups (p<0.05).
8. Discussion
8.1. Day 1 behavior
In Experiment 1, as expected from the results of several prior studies with predators or some of their odors, rats exposed acutely to ferret odor avoided the predator stimulus and displayed more risk-assessment behaviors as compared to control odor exposed rats. Avoidance and risk assessment are the most common behaviors seen with predator odor [3,9,13,16,17,25]. Ferret odor exposed rats also entered the defensive withdrawal chamber less than control odor exposed rats, even if they did not differ in the time spent in the chamber, again suggesting an overall reduction in active locomotor activity. The behavioral differences in this experiment replicate those previously found with ferret odor exposure in a defensive withdrawal paradigm [4], and studies using cat odors in similar paradigms [16,27]. However, immobility or “freezing” behavior was not clearly observed in response to ferret odor in the present experiments or in previous studies using ferret odor [4]. It is possible that the rats were immobile inside the defensive withdrawal chamber, but because the rats could not be observed in the metal chamber, this could not be confirmed.
The behavioral differences between ferret odor exposed rats and control odor exposed rats found on day 1 were similar to those found on day 1 of Experiment 2, when rats were not previously exposed to the behavior apparatus before testing. However, and presumably because of the lack of pre-exposure and familiarity to the behavior apparatus, the rats generally were less active and spent more time in the defensive withdrawal chamber in Experiment 2. Thus, the acute effects of predator odors can be observed against at least two levels of initial emotional background states.
8.2. Habituation
To determine if the behavioral responses to ferret odor habituate, a group of rats was repeatedly exposed to ferret odor towels in the behavior apparatus. Compared to control odor exposed rats, rats repeatedly exposed to ferret odor failed to display habituation to most of the behaviors measured over 7 days of exposure. The ability of ferret odor to elicit responses that do not habituate after repeated exposures suggests that it is a highly potent stimulus. Using a similar defensive withdrawal paradigm, McGregor and Dielenberg found that even if the avoidance of a worn cat collar stimulus by rats did not habituate over 10 days of repeated exposures, the “hiding” or time spent in withdrawal chamber response did habituate after 5 days of exposures [18]. It is possible that ferret odor, even if only a partial predator stimulus, is a more potent unconditioned stimulus than cat odor and thus makes the rats' hiding responses more resistant to habituation. Behavioral responses to live predators are very resistant to habituation. Blanchard et al. report that defensive crouching or freezing in response to a live cat in a cage above the rat does not habituate over 20 daily 60 min sessions [2]. The interesting conclusion of the present, and previously reported studies employing repeated live predators or some stimuli associated with them, is that they don't readily habituate, if at all.
Blanchard et al. also found higher basal levels of corticosterone in cat-exposed rats after 20 daily exposures, suggesting lack of habituation [2]. However, because corticosterone was not measured at the beginning of this study, this cannot easily be distinguished from a possible cumulative sensitization effect of repeated cat exposures. The results of Experiment 3 suggest that 9 repeated daily exposures to ferret odor does not reduce hypothalamo–pituitary–adrenal (HPA) axis response. But, the levels of ACTH and corticosterone in the control group were higher on day 9 than days 1 and 4. This could possibly be due to the repeated tail nick procedure. Replication of this experiment using intravenous blood draws with little disturbance of the rat would clarify this finding. The present behavioral and endocrine findings combined with previous literature confirm that predators and some of their associated odors are remarkably resistant to habituation following repeated exposures [12].
8.3. Conditioning
There have been several reports of rapid (1 exposure) conditioning with live cats and cat odor [10,15,25,27,31]. Blanchard et al. report that after one 10 min exposure to a cat odor block stimulus, rats 24 h later exhibit defensive behaviors (stretch attend and approach duration) and avoidance of a no-odor block during an extinction trial [15]. The rats put back into the same context as the initial cat odor exposure with a no-odor block stimulus (cue) during the extinction trial exhibited the most defensive or “risk assessment” behaviors, suggesting both context and cue conditioning. Dielenberg et al. also report avoidance of a no-odor cat collar and hiding in a defensive withdrawal chamber during an extinction trial one day after a 20 min exposure to a worn cat collar in the same context [8]. In Experiment 1, one or seven repeated exposures to ferret odor did not provide any strong evidence of conditioning to any of the behaviors measured during extinction trials. This suggests that the ferret odor on the towel did not become associated with the towel (cue) or behavior apparatus (context). Before reaching this conclusion, however it was important to test the possibility that conditioning to ferret odors may have been hampered by the contextual pre-exposure manipulation. Although other researchers examining conditioning with live cats or cat odor have also acclimated the rats to the behavior apparatus before testing and still found rapid conditioning [10,15,26], it is possible that pre-exposure to the context could cloud fear conditioning due to latent inhibition [32]. However, even without context pre-exposure, only very weak fear conditioning was observed to only a subset of behavioral measures in the present study.
2,5-dihydro-2,4,5-trimethylthiazoline (TMT), which is a component of fox feces, has also been used as a predator odor stress model [33–40]. Researchers have found that the freezing or immobility response elicited by TMT exposure does not habituate over several repeated exposures [40,41]. They have also reported that TMT does not elicit context conditioning [10,40,41], like ferret odor in the present studies. However, other researchers have suggested that TMT is aversive because of noxious rather than predatory characteristics and thus does not lead to conditioned defensive behavior in extinction trials [10,15,26,31,42,43]. The ferret skin/fur odor used in the present experiment on the other hand is like cat odor and is barely detectable by humans. The scent normally associated with ferrets is from their anal scent glands [44–47]. The anal scent gland secretions however do not produce the endocrine and behavioral stress responses found with the fur/skin odor [4]. Ferret odor exposure also produces a c-fos mRNA expression pattern more similar to cat odor than to TMT exposure [4,42,48]. At this time therefore, it is unclear why cat, ferret, and TMT odors display different potentials as unconditioned stimuli in Pavlovian conditioning paradigms.
8.4. Sensitization
A sensitized endocrine response to ferret odor was obtained at the end of Experiment 1 in the group of rats that had received only a single prior ferret odor exposure, as compared to rats receiving ferret odor for the first time or repeatedly (seven times). This endocrine response difference between the rats receiving one versus seven ferret odor exposures is remarkable. This finding suggests that although little behavioral and endocrine habituation takes place to repeated predator odor exposures, repeated presentation may yet reduce sensitization, or produce desensitization. This possibly is an additional instance demonstrating the distinction between habituation and sensitization processes [11], and could have important implications for the treatment of some stress-related disorders. A single session of footshock stress has been shown to sensitize HPA axis activation and increase defensive behaviors [28,49–53]. It is possible that the initial ferret odor exposure acted in a way similar to footshock. Adamec and colleagues reported that a 5-min unprotected exposure of a rat to a cat produced long-term sensitization of anxiety-like behavior in the rat [54–57]. In the present study, it is interesting to note that the ACTH and corticosterone responses in the group of rats repeatedly exposed to ferret odor did not significantly differ from the responses of the control rats. Reasons for this are unclear and need to be examined further. Plata-Salaman et al. reported a similar plasma corticosterone response (24.4±2.8 μg/dl) to live ferrets after 30 days of ferret exposures, as the present study found in the habituation group (24.8±2.8 μg/dl) and control group (32.0±3.7 μg/dl) after ferret odor exposure [6]. This further suggests that ferret odor is a very potent unconditioned stimulus.
8.5. Conclusions
In summary, exposure to ferret odor elicits behavioral avoidance, risk assessment behavior, and plasma ACTH and corticosterone release. These behavioral responses to ferret odor did not habituate over 7 repeated exposures in a defensive withdrawal paradigm. And, repeated home cage exposures to ferret odor also suggest that the endocrine response does not rapidly habituate. One previous exposure to ferret odor also significantly increased the plasma ACTH and corticosterone response to an additional delayed ferret odor exposure in a different context. However, in the same defensive withdrawal context, previous ferret odor exposure did not lead to reliable behavioral conditioning effects during extinction trials. Future experiments need to be designed to determine why this potent unconditioned stimulus does not readily produce clear associative learning, and whether repeated exposure to such a non-habituating stimulus reduces sensitization that is otherwise obtained after a single exposure.
References
- 1.Anisman H, Lu ZW, Song C, Kent P, McIntyre DC, Merali Z. Influence of psychogenic and neurogenic stressors on endocrine and immune activity: differential effects in fast and slow seizing rat strains. Brain Behav Immun. 1997;11(1):63–74. doi: 10.1006/brbi.1997.0482. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63(4):561–9. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 3.File SE, Zangrossi H, Jr, Sanders FL, Mabbutt PS. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav. 1993;54(6):1109–11. doi: 10.1016/0031-9384(93)90333-b. [DOI] [PubMed] [Google Scholar]
- 4.Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119(1):280–92. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre DC, Kent P, Hayley S, Merali Z, Anisman H. Influence of psychogenic and neurogenic stressors on neuroendocrine and central monoamine activity in fast and slow kindling rats. Brain Res. 1999;840(1–2):65–74. doi: 10.1016/s0006-8993(99)01771-0. [DOI] [PubMed] [Google Scholar]
- 6.Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, et al. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1beta system, tumor necrosis factor-alpha, transforming growth factor-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Bull. 2000;51(2):187–93. doi: 10.1016/s0361-9230(99)00204-x. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard RJ, Blanchard DC. Defensive reactions in the albino rat. Learn Motiv. 1971;21:351–62. [Google Scholar]
- 8.Dielenberg RA, Arnold JC, McGregor IS. Low-dose midazolam attenuates predatory odor avoidance in rats. Pharmacol Biochem Behav. 1999;62(2):197–201. doi: 10.1016/s0091-3057(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 9.McGregor IS, Dielenberg RA. Differential anxiolytic efficacy of a benzodiazepine on first versus second exposure to a predatory odor in rats. Psychopharmacology Berl. 1999;147(2):174–81. doi: 10.1007/s002130051158. [DOI] [PubMed] [Google Scholar]
- 10.McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129(1–2):1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- 11.Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–50. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard DC, Blanchard RJ. Ethoexperimental approaches to the biology of emotion. Annu Rev Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:S3–14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard RJ, Yang M, Li CI, Gervacio A, Blanchard DC. Cue and context conditioning of defensive behaviors to cat odor stimuli. Neurosci Biobehav Rev. 2001;25(7–8):587–95. doi: 10.1016/s0149-7634(01)00043-4. [DOI] [PubMed] [Google Scholar]
- 16.Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25(7–8):597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 17.Zangrossi H, Jr, File SE. Behavioral consequences in animal tests of anxiety and exploration of exposure to cat odor. Brain Res Bull. 1992;29(3–4):381–8. doi: 10.1016/0361-9230(92)90072-6. [DOI] [PubMed] [Google Scholar]
- 18.Dielenberg RA, McGregor IS. Habituation of the hiding response to cat odor in rats (Rattus norvegicus) J Comp Psychol. 1999;113(4):376–87. doi: 10.1037/0735-7036.113.4.376. [DOI] [PubMed] [Google Scholar]
- 19.File SE, Zangrossi H, Jr, Sanders FL, Mabbutt PS. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav. 1993;54(6):1109–11. doi: 10.1016/0031-9384(93)90333-b. [DOI] [PubMed] [Google Scholar]
- 20.Andrews N, Barnes NM, Steward LJ, West KE, Cunningham J, Wu PY, et al. A comparison of rat brain amino acid and monoamine content in diazepam withdrawal and after exposure to a phobic stimulus. Br J Pharmacol. 1993;109(1):171–4. doi: 10.1111/j.1476-5381.1993.tb13548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogg S, File SE. Responders and nonresponders to cat odor do not differ in other tests of anxiety. Pharm Biochem Behav. 1994;49(1):219–22. doi: 10.1016/0091-3057(94)90479-0. [DOI] [PubMed] [Google Scholar]
- 22.Apfelbach R. Instinctive predatory behavior of the ferret (Putorius putorius furo L.) modified by chlordiazepoxide hydrochloride (Librium) Psychopharmacology. 1978;59(2):179–82. doi: 10.1007/BF00427754. [DOI] [PubMed] [Google Scholar]
- 23.Rusiniak KW, Gustavson CR, Hankins WG, Garcia J. Prey-lithium aversions. II: laboratory rats and ferrets. Behav Biol. 1976;17(1):73–85. doi: 10.1016/s0091-6773(76)90287-x. [DOI] [PubMed] [Google Scholar]
- 24.Muzur JE. Learning and behavior. 3rd. Englewood Cliffs, New Jersey: Prentice Hall; 1994. [Google Scholar]
- 25.Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103(1):70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117(2):360–8. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard RJ, Kelley MJ, Blanchard DC. Defensive reactions and exploratory behavior in rats. J Comp Physiol Psychol. 1974;87:1129–33. [Google Scholar]
- 28.Bruijnzeel AW, Stam R, Wiegant VM. Effect of a benzodiazapine receptor agonist and corticotropin-releasing hormone receptor antagonists on long-term foot-shock-induced increase in defensive withdrawal behavior. Psychopharmacology. 2001;158:132–9. doi: 10.1007/s002130100863. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi LK, Kalin NH, Baker EW. Corticotropin-releasing factor antagonist attenuates defensive-withdrawal behavior elicited by odors of stressed conspecifics. Behav Neurosci. 1990;104(2):386–9. doi: 10.1037//0735-7044.104.2.386. [DOI] [PubMed] [Google Scholar]
- 30.Masini CV, Sauer S, Campeau S. Associative and non-associative responses to ferret odor. Abstract from International Behavioral Neuroscience Society Meeting; Key West, FL. June 16–20; 2004. [Google Scholar]
- 31.Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1177–85. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Lubow RE, Moore AU. Latent inhibition: the effect of nonreinforced pre-exposure to the conditioned stimulus. J Comp Physiol Psychol. 1959;52:415–9. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- 33.Falconer EM, Galea LA. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975(1–2):22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- 34.Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23(1):23–8. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25(25):5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes MM, Galea LA. Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behav Neurosci. 2002;116(1):160–8. [PubMed] [Google Scholar]
- 37.Hotsenpiller G, Williams JL. A synthetic predator odor (TMT) enhances conditioned analgesia and fear when paired with a benzodiazepine receptor inverse agonist (FG-7142) Psychobiology. 1997;25(1):83–8. [Google Scholar]
- 38.Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000;864:146–51. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- 39.Redmond AJ, Morrow BA, Elsworth JD, Roth RH. Selective activation of the A10, but not A9, dopamine neurons in the rat by predator odor, 2,5,-dihydro-2,4,5-trimethylthiazoline. Neurosci Lett. 2002;328:209–12. doi: 10.1016/s0304-3940(02)00566-9. [DOI] [PubMed] [Google Scholar]
- 40.Wallace KJ, Rosen JB. Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci. 2000;114(5):912–22. doi: 10.1037//0735-7044.114.5.912. [DOI] [PubMed] [Google Scholar]
- 41.Endres T, Apfelbach R, Fendt M. How does fox odor influence the behavior of naïve rats?. Abstract from International Behavioral Neuroscience Society Meeting; Key West, FL. June 16–20; 2004. [Google Scholar]
- 42.Day HEW, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5,-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025(1–2):139–51. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 43.Lowry CA, Kay LM. Predator and non-predator odors: similarities in spectral and behavioral response patterns. Presentation at the International Behavioral Neuroscience Society Satellite; Key West, FL. June 16–20; 2004. [Google Scholar]
- 44.Clapperton BK. Scent-marking behaviour of the ferret Mustela furo L. Anim Behav. 1989;38:436–46. [Google Scholar]
- 45.Clapperton BK, Minot EO, Crump DR. An olfactory recognition system in the ferret Mustela furo L. (Carnivora: Mustelidae) Anim Behav. 1988;36:541–53. [Google Scholar]
- 46.Crump DR. Anal gland secretion of the ferret (Mustela putorius forma furo) J Chem Ecol. 1980;6(4):837–44. doi: 10.1007/BF01020673. [DOI] [PubMed] [Google Scholar]
- 47.Woodley SK, Baum MJ. Effects of sex hormones and gender on attraction thresholds for volatile anal scent gland odors in ferrets. Horm Behav. 2003;44(2):110–8. doi: 10.1016/s0018-506x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 48.Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104(4):1085–97. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 49.Bruijnzeel AW, Stam R, Compaan JC, Wiegant VM. Stress-induced sensitization of CRH-ir but not P-CREB-ir responsivity in the rat central nervous system. Brain Res. 2001;908:187–96. doi: 10.1016/s0006-8993(01)02646-4. [DOI] [PubMed] [Google Scholar]
- 50.Caggiula AR, Antelman SM, Aul E, Knopf S, Edwards DJ. Prior stress attenuates the analgesic response but sensitizes the corticosterone and cortical dopamine responses to stress 10 days later. Psychopharmacology. 1989;99:233–7. doi: 10.1007/BF00442814. [DOI] [PubMed] [Google Scholar]
- 51.Levine S, Madden J, Conner RL, Moskal JR, Anderson DC. Physiological and behavioral effects of prior aversive stimulation (preshock) in the rat. Physiol Behav. 1973;10:417–67. doi: 10.1016/0031-9384(73)90207-2. [DOI] [PubMed] [Google Scholar]
- 52.Pitman DL, Ottenweller JE, Natelson BH. Effect of stressor intensity on habituation and sensitization of glucocorticoid responses in rats. Behav Neurosci. 1990;104(1):28–36. doi: 10.1037//0735-7044.104.1.28. [DOI] [PubMed] [Google Scholar]
- 53.Van Dijken HH, De Goeij DCE, Sutanto W, Mos J, De Kloet ER, Tidlers FJH. Short inescapable stress produces long-lasting changes in the brain–pituitary–adrenal axis of adult male rats. Neuroendocrinology. 1993;58:57–64. doi: 10.1159/000126512. [DOI] [PubMed] [Google Scholar]
- 54.Adamec RE, Burton P, Shallow T, Budgell J. NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure—implications for anxiety associated with post-traumatic stress disorder. Physiol Behav. 1999;65(4–5):723–37. doi: 10.1016/s0031-9384(98)00226-1. [DOI] [PubMed] [Google Scholar]
- 55.Adamec RE, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals—implications for understanding and treating affective disorder following traumatic stress in humans. Neurosci Biobehav Rev. 1998;23(2):301–18. doi: 10.1016/s0149-7634(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 56.Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav. 1993;54:101–9. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- 57.Adamec RE, Shallow T, Budgell J. Blockade of CCK-B but not CCK-A receptors before and after the stress of predator exposure prevents lasting increases in anxiety-like behavior: implications for anxiety associated with posttraumatic stress disorder. Behav Neurosci. 1997;111(2):435–49. doi: 10.1037//0735-7044.111.2.435. [DOI] [PubMed] [Google Scholar]