Abstract
A significant portion of the transcriptome in mammals, including the PAR bZIP transcription factors DBP, HLF and TEF, is under circadian clock control. In this issue, Gachon and colleagues show that disruption of these three genes in mice alters the gene expression patterns of many proteins that are involved in drug metabolism and responses to xenobiotic agents in liver and kidney. Triple mutant mice have severe physiological deficits, including increased hypersensitivity to xenobiotic agents and premature aging, highlighting the profound effect the circadian clock has on this important response system.
Humans normally only notice their circadian clocks when they are disrupted – when struggling to stay awake upon arrival in a new time zone, or when trying to perform optimally at a difficult job when working the night shift. At times like this, this internal clock seems to be an inconvenience, making it difficult to function optimally when out of phase with one’s environment. However, it has become increasingly clear that circadian clocks control a vast array of physiological functions and behaviors that are critically important to an organism’s well being. This has come into sharper focus in recent years as genetic disruption of circadian systems has revealed a number of serious health consequences (Hastings et al., 2003). In this issue of Cell Metabolism, Gachon et al demonstrate that loss of three circadian-controlled PAR bZIP transcription factors in mice causes a disruption of a rhythmic transcriptional program that regulates circadian detoxification. These mice exhibit hypersensitivity to xenobiotic compounds and display signs of premature aging, providing a compelling example of the importance of the circadian system.
Circadian clocks are found in a wide spectrum of organisms ranging from cyanobacteria to humans, with many well-conserved properties [reviewed in (Bell-Pedersen et al., 2005)]. In mammals, the circadian oscillator consists of a core negative feedback loop in which the transcription factors CLOCK and BMAL1 activate the Period (Per1, Per2) and Cryptochrome (Cry1, Cry2) genes via E-box enhancers in their promoters [Figure 1; reviewed in (Lowrey and Takahashi, 2004)]. The products of these genes form complexes with each other and with other proteins and eventually translocate into the nucleus and repress the CLOCK/BMAL1 complex, shutting off their own transcription. This primary negative feedback loop is augmented by an interlocking loop in which CLOCK/BMAL1 also drive transcription of other transcription factors (REV-ERB α and RORA) that act to drive rhythmic transcription of the Bmal1 gene. The circadian mechanism is cell autonomous, and the majority of cells and tissues in the body contain circadian oscillators. At the organismal level, temporal organization is achieved by a hierarchical order in which a circadian pacemaker in the suprachiasmatic nucleus (SCN) synchronizes and coordinates peripheral tissue oscillators throughout the body (Yoo et al., 2004).
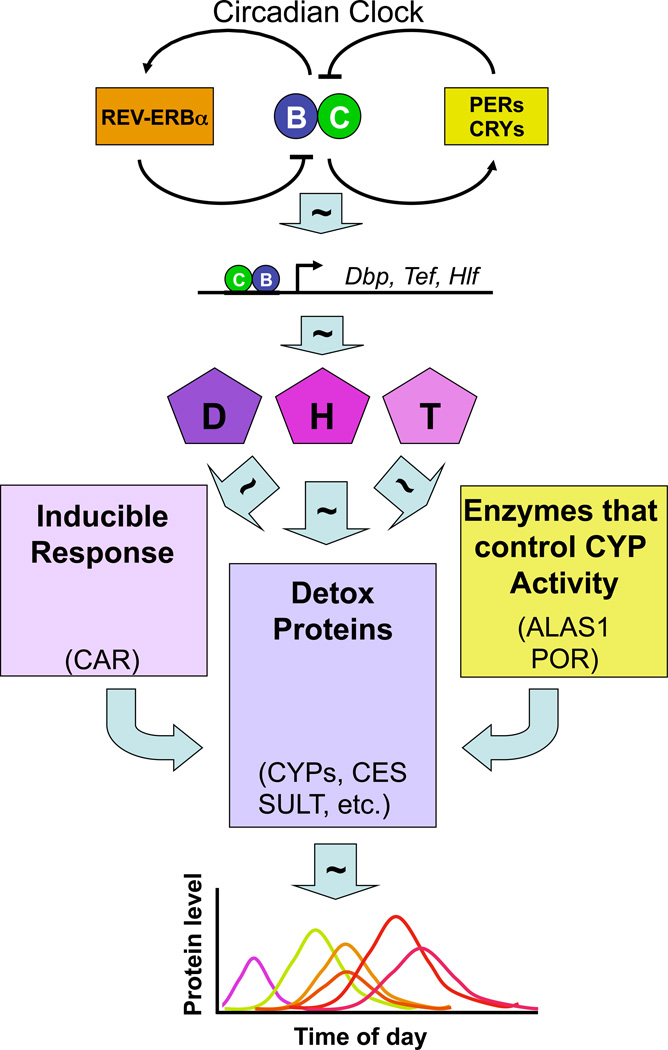
Figure 1. The circadian oscillator drives a cascade of temporally coordinated rhythmic gene expression that is necessary for a proper response to xenobiotics.
The core circadian oscillator mechanism (top) is composed of two interlocking loops which produce rhythmic activity of the heterodimeric transcription factor composed of CLOCK (C) and BMAL1 (B). CLOCK and BMAL1 drive rhythmic gene expression of “output” genes, including those encoding the PAR-bZIP transcription factors DBP (D), HLF (H) and TEF(T). These transcription factors form homo- and heterodimers and activate other genes rhythmically, including those involved in the response to xenobiotics. These proteins drive expression of detoxification genes, including several Cytochrome P450s (CYPs), sulfotransferases (SULT), carboxylesterase (CES), and others (center box). In addition, these transcription factors also regulate genes encoding a retinoid receptor (CAR) that regulates detoxification genes in a xenobiotic-inducible manner (right box), and those encoding enzymes (ALAS1 and POR) involved in providing heme and electrons important for the activation of cytochrome P450s (right box). The net result of this cascade is the appropriately timed production of the many proteins needed for xenobiotic responsiveness as represented in the graph (bottom).
So, how do circadian clocks composed of interlocking feedback loops control these various output pathways? Microarray analyses have shown that ~3–10% of expressed transcripts are under circadian regulation [reviewed in (Lowrey and Takahashi, 2004)]. In the liver, basic cellular pathways such as glycolysis, fatty acid metabolism, cholesterol biosynthesis, and xenobiotic and intermediate metabolism are under circadian regulation. Importantly, rate-limiting steps in these various pathways are key sites of circadian control, highlighting the fundamental role that circadian clocks play in cellular and organismal physiology (Panda et al., 2002). Gachon et al. provide new insight into the complexities of circadian gene regulatory networks using genetic and biochemical approaches (Gachon et al., 2006). In this paper, they examine the role of three PAR-domain basic leucine zipper (PAR bZip) transcription factors in regulation of rhythmic gene expression in liver. One of these proteins, DBP, has been definitively shown by this group to be a direct transcriptional target of CLOCK/BMAL1 (Ripperger and Schibler, 2006; Ripperger et al., 2000). The other two, TEF and HLF, are reported here to also be under similar control. These three proteins can form homo- or heterodimers and activate transcription of genes containing the appropriate PAR response element (PARRE).
In order to evaluate how these rhythmic transcription factors contribute to circadian function, all three PAR bZIP genes were inactivated by gene targeting in mice. The phenotypes of these mice, as well as all combinations of double knock-outs, however, were mild. Only after the heroic effort of generating triple knock-outs (H−/−/D−/−/T−/−) were strong phenotypes observed, including epileptic seizures in early life and advanced aging after 9 months of life (Gachon et al., 2004).
Because the liver and kidney are the only tissues known to express all three of these transcription factors, these investigators examined the gene expression changes in these two tissues in the triple knockout mice. After profiling the mRNAs in these tissues, they discovered that many of the genes that were down-regulated in both tissues in the triple knockouts encoded proteins that are involved in metabolism of xenobiotic agents. These include genes encoding members of the cytochrome P450 family (Cyp4a, Cyp2c), a nuclear receptor (constitutive adrostane receptor; CAR) which senses xenobiotic compounds and activates transcription of several detoxification enzymes, sulfotransferases, and drug transporter family members, among others (Figure 1).
Interestingly, these differentially expressed mRNAs had a range of profiles, with some of them normally rhythmic (but with various phases and amplitudes) and some of them normally constitutive. They also were affected to varying degrees by the loss of the three transcription factors. Some of these genes had profiles that were consistent with direct activation by HLF/DBP/TEF and two of these genes (Ces3 and AK3l1) were shown to contain PARRE sequences in their promoters that bound these PAR transcription factors in vitro. However, a number of other mRNAs had profiles that were incompatible with direct regulation by these proteins, suggesting that both direct and indirect regulation was generating this complex pattern of gene expression.
Finally, the functional relevance of the xenobiotic gene profile differences were tested in vivo by challenging the triple knockouts with pentobarbitol and chemotherapeutic agents. The wildtype mice showed pronounced circadian rhythms in response to pentobarbitol, with much faster clearance at night than in the day, while the triple knockout mice had severe deficits in the clearance of the pentobarbitol at all times of day. Likewise, two chemotherapeutic agents (mitoxantrone and cyclophosphamide) had increased toxicity in the triple knockout animals. Interestingly, an increase in morbidity to cyclophosphamide has also been found in Clock and Bmal1 mutant mice providing another circadian connection (Gorbacheva et al., 2005). Because Dbp, Tef and Hlf are targets of CLOCK and BMAL1, these PAR-bZIP proteins provide a potential causative link.
Despite the assumption for several decades that circadian modulation of the pharmacokinetic properties of therapeutic agents should be physiologically significant, direct evidence has rarely been available. The results by Gachon et al. provide an important example of the fundamental role that circadian clocks play at the cellular and metabolic level and highlight their dire consequences when disrupted at the organismal level. A deeper understanding of circadian detoxification mechanisms provides a rational basis for optimizing the efficacy of pharmaceutical agents whose toxicity and side effects should be reduced by delivery at optimal times of day. Perhaps one day, both the timing and dose of drug administration will become routine in clinical practice.
References
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Fonjallaz P, Damiola F, Gos P, Kodama T, Zakany J, Duboule D, Petit B, Tafti M, Schibler U. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev. 2004;18:1397–1412. doi: 10.1101/gad.301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metabolism. 2006 doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, Takahashi JS, Antoch MP. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A. 2005;102:3407–3412. doi: 10.1073/pnas.0409897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian Circadian Biology: Elucidating Genome-Wide Levels of Temporal Organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14:679–689. [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]