Abstract
Hepatic angiomyolipoma (AML), a rare benign mesenchymal tumour, is characterised by the presence of mature adipose tissue, smooth‐muscle cells and thick‐walled blood vessels. Increasing attention to hepatic AMLs has led to the discovery that sufficient proportions of fat often allow for definite diagnoses preoperatively. However, the proportion of fatty tissue in these tumours is highly variable. One case of hepatic AML is reported, where the amount of fat was <1%. In this case, the viral hepatitis markers, including hepatitis B antigen and anti‐hepatitis C virus antibody, were negative. The serum α‐fetoprotein level was 3.4 ng/ml and in the normal range. Abdominal ultrasonography showed a hypoechoic mass measuring 5 cm in diameter and without an obvious capsule in the left lobe of the liver. A dynamic computed tomography scan showed a well‐defined and slightly enhanced mass in the medial segment of the left lobe of the liver. Angiography showed that the mass was hypervascular in character. As hepatocellular carcinoma was highly suspected from these preoperative image studies, a left lobectomy was carried out. Microscopically, the amount of fat was too low to establish a diagnosis of hepatic AML. However, positive homatropine methylbromide 45 immunoreactivity of the smooth‐muscle cells seemed to assist in arriving at the diagnosis.
Hepatic angiomyolipoma (AML) is a rare benign mesenchymal liver tumour, mainly composed of blood vessels and smooth muscle, and fat cells in varying amounts. The detection of tumours has increased markedly with refined imaging modalities. In most cases, fat content is sufficient to produce a characteristic image that allows for definite differentiation from the hepatocellular carcinoma preoperatively.1 However, the proportion of fatty tissue in these tumours is highly variable. Numerous reports have indicated that homatropine methylbromide 45 (HMB45), one of the melanoma markers, produces positive results consistently in AMLs.2 Thus, HMB45 has become a promising tool in the diagnosis of hepatic AMLs, especially in cases with trace amounts of fat.
We describe one case of hepatic AML where the fatty part was detected in small amounts. Its low fat content did not allow for correct diagnosis before surgery, even causing diagnostic problems in pathology. Eventually, HMB45 was used to settle the issue of diagnosis. Moreover, we review several articles from the literature and attempt to elucidate the importance of HMB45 in hepatic AMLs with minimal fat.
Case report
A 32‐year‐old man was referred to the Department of Surgery at Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, for further evaluation, as an abdominal ultrasound examination showed a liver tumour during a health check‐up. He did not have any abdominal discomfort. No palpable mass was found over the upper right quadrant of his abdomen during physical examination. His medical history showed no history of tuberous sclerosis. On routine blood examination, his blood counts were found to be normal. Serum chemistries were notable only for a slight increase of 258 IU/l (normal 70–237 IU/l) in alkaline phosphatase and 79 U/l (normal 3–30 U/l) in γ‐glutamyltransferase. In addition, liver function tests, including serum levels of aspartate transaminase, alanine transaminase, bilirubin and albumin, and prothrombin time, were all normal. Furthermore, serum viral hepatitis markers, including hepatitis B antigen and anti‐hepatitis C virus antibody, showed negative results. The level of serum α‐fetoprotein did not extend beyond the normal limit (3.4 ng/ml).
Abdominal ultrasonography showed a well‐defined, homogeneous and hypoechoic mass, with a maximum diameter of 5.4 cm in the medial segment of the left part of the liver. No capsule was noticed surrounding the tumour. A plain computed tomography scan showed a well‐circumscribed and iso‐attenuating tumour over the left lobe of the liver (fig 1A). In the dynamic phase, the tumour was slightly enhanced by the contrast media (fig 1B). In the late phase, the tumour returned to an iso‐attenuating state. Angiography showed that the tumour was hypervascular in character (fig 1C). With these image findings, we strongly suspected the existence of hepatocellular carcinoma preoperatively. Accordingly, a left hepatic lobectomy was carried out to remove the tumour. A full abdominal exploration showed a tumour outgrowth from the medial segment of the left liver, but no evidence of distant metastasis. Postoperatively, the patient's recovery course was uneventful and there has been no evidence of recurrence for 38 months.
Figure 1 (A) A plain computer tomography scan showing a well‐circumscribed and iso‐attenuating lesion in the medial segment of the left lobe of the liver (arrow). (B) After injection of contrast material, slight enhancement of the lesion is noted (arrow). (C) Angiography showing the hypervascular character of the lesion (arrow).
Pathological findings
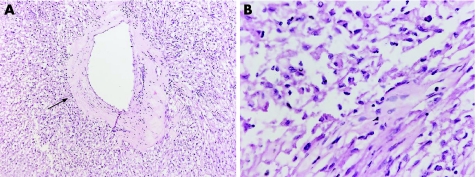
The tumour was clearly demarcated from the surrounding liver tissue in gross appearance, and there was no fibrous capsule. The surgical margins were free of tumour. Microscopically, the tumour was composed almost exclusively of smooth‐muscle cells (SMCs) and a small number of blood vessels. The blood vessels appeared dilated and thick walled (fig 2A). We were initially unable to detect the proportion of fat in the tumour in 3–4 different low‐power fields. Further careful examination showed that the fat existed in minimal amounts (<1% of the tumour lesion) in the high‐power field. Extramedullary haematopoiesis was not present in this case.
Figure 2 (A) Microscopically, the tumour is composed almost exclusively of smooth‐muscle cells (SMCs) and a small number of thick‐walled blood vessels (arrow). The fat component is almost undetected in this tumour (haematoxylin and eosin; ×20). (B) The tumour consists of two types of SMCs: epithelioid and spindle shaped. The epithelioid type predominates and shows trabecular growth pattern. The spindle‐shaped type is scattered in low proportions (haematoxylin and eosin; ×80).
Immunohistochemical stains for HMB45 and smooth‐muscle actin (SMA) were positive in the lesion. Focal staining was seen for desmin. Tumour cells were negative for S‐100, keratin, crystalline egg albumin, hepatocytes and α‐fetoprotein. As for the SMC component, the tumour was predominantly composed of epithelioid cells, with a low proportion of spindle‐shaped cells (fig 2B). These epithelioid cells were polygonal, with clear and abundant cytoplasm. They showed a trabecular pattern in some areas. Spindle‐shaped cells showed filamentous and eosinophilic cytoplasm with ovoid nuclei. Both epithelioid and spindle‐shaped SMCs showed strong diffuse staining for SMA and HMB45 (fig 3A,B). Finally, a diagnosis of hepatic angiomyolipoma was made.
Figure 3 (A) Staining of smooth‐muscle actin (SMA) confirms the existence of the smooth‐muscle cell component (SMA stain; ×40). (B) Immunohistochemistry shows strong cytoplasmic staining with human melanoma‐specific monoclonal antibodies homatropine methylbromide 45 (HMB45) in a perinuclear pattern (HMB45, ×40).
Discussion
AML, an unusual lesion of harmatomatous and neoplastic nature, mainly occurs in the kidneys. The tumour is typically comprised of blood vessels, SMCs and fat, but any single component can predominate. It is shown that the SMCs and fat cells of AMLs are derived from the perivascular epithelioid cells (PECs) and AMLs are allocated to the family of PEC tumours.3,4 AML is rarely encountered in other organs, including the liver, retroperitoneum, spleen, lungs, skin, and so on. Since Ishak5 described the first hepatic AML in 1976, the advent of refined imaging modalities had led to the ever‐expanding list of hepatic AMLs. Although 40–50% of patients with renal AMLs have been found to have tuberous sclerosis, the association has decreased to 10% for hepatic AMLs.6
Haemorrhages and ruptures are known complications of renal angiomyolipomas and can be occasionally expected to occur in hepatic AML. In fact, most cases of hepatic AML are either asymptomatic or produce non‐specific symptoms. Thus, it seems difficult to make a correct diagnosis preoperatively. In Taiwan, clinical staff often face the diagnostic challenge of differentiating hepatocellular cancers from other liver tumours before surgery. Several reports have described situations in which a possible misdiagnosis of a hepatic AML as a hepatocellular carcinoma or another liver tumour has occurred.7 For example, Sajima et al8 reviewed 48 cases of hepatic AML, of which only 10 cases were correctly diagnosed before surgery.
According to the predominant components, AMLs can be categorised into several types, including mixed (the most common type), lipomatous (⩾70% fat), myomatous (⩽10% fat) and angiomatous. A classic mixed AML shows all tissue components. Regarding the fat component, echo images and computed tomography can provide typical pictures, allowing for a correct diagnosis. Such tumours often appear as hyperechoic masses with low density on a computed tomography scan, usually <−20 HU in attenuation values.8,9 As a result, most hepatic AMLs can be confidently diagnosed in the context of sufficient fat content on imaging modalities. However, other fat‐containing tumours, such as hepatic adenomas or hepatocellular carcinomas with fatty metamorphosis, may be considered in the differential diagnosis of AMLs.
The fat proportion, however, is highly variable from patient to patient, ranging from 5% to >50%. This can cause diagnostic problems if the amount of fat is too low to represent characteristic features in images. Although fatty tissue exists less often in low proportions in hepatic AML, such cases are still reported occasionally. In the literature, Goodman and Ishak10 first reported five cases in which the fat component occupied <10% of the tumour, on average. In 1996, Terris et al9 reported four cases of AML in which the low content of fat failed to appear as typical images before surgery. Tsui et al6 also presented 10 cases of tumour with low fat content and showed that these tumours showed widely variable patterns in morphology. In our case, we were initially unable to detect the fat proportion in the tumour in echo images and on computed tomography scans, as well as microscopically in 3–4 different low‐power fields. Eventually, the fat content was seen to be minimal (<1% of the tumour lesion) when examined in the high‐power field.
In most cases, the histological diagnosis is made straightforward by the presence of adipose tissue, disorganised SMCs and aberrant vascular structures in the same lesion. However, several difficulties exist in histological diagnosis. One reason is that SMCs always assume the predominant part in AML with low fat content, regardless of its renal or hepatic origin. Because SMCs in AMLs usually appear in a heterogeneous pattern, the differential diagnosis often confuses pathologists. Regarding morphology, Nonomura et al11 classified the SMCs of the AML into four types: spindle shaped, intermediate, epithelioid and pleomorphic. For example, monotypic epithelioid AML is characterised by the presence of epithelioid, spindle‐shaped and giant cells with no or only minimal lipomatous tissue.12 Although each type may cause different diagnostic problems, the positive immunoreactivity for HMB45 is shown in these four SMC types of AML. Moreover, other SMC tumours, including leiomyoma and leiomyosarcoma, do not show the positive immunoreactivity of HMB45. Given that HMB45 is a consistent finding in AMLs, we can clearly differentiate AML from other SMC tumours. As mentioned above, most hepatic AMLs can be diagnosed preoperatively in the context of sufficient fat content. Early and prolonged enhancement with a special pattern of time–density or intensity curve on a dynamic study of computer tomography and magnetic resonance imaging may be helpful for a correct diagnosis.13 However, in our case, we were unable to detect the proportion of fat in the tumour on imaging modalities and on pre‐biopsy diagnoses. Therefore, a comprehensive immunohistochemical panel on this tumour may facilitate the correct diagnosis.
HMB45, a monoclonal antibody, was first generated by Gown et al in 1986.14 Over the years, its positive immunoreactivity has been elucidated as a melanocyte‐associated marker. For practical purposes, a positive reaction with HMB45 is therefore suggestive of active melanosome formation and melanocytic differentiation. In addition to the lesions associated with pigmentation, one set of tumours without obvious pigmentation has been found to consistently manifest HMB45 immunoreactivity. These lesions, collectively allocated to the family of perivascular epitheliod carcinomas, include clear‐cell tumours of the lung, AML and lymphangiomyomatosis. Despite the absence of pigmentation, some authors have reported ultrastructural evidence of melanogenesis in these cells of AML, thus providing a rationale for HMB45 positivity.15 Ren et al16 reported 26 cases of hepatic AML, and the rate of positive HMB45 immunostaining was 100% (26/26). Zhong and Ji17 reviewed 14 cases of hepatic AML that were initially misdiagnosed as hepatocellular carcinoma. Their final diagnosis was reached through the positive result of HMB45 immunostaining.17 In addition, AMLs are shown to be SMA immunoreactive and, to a lesser degree, desmin immunoreactive. Although AMLs presented with pleomorphic cells and a low proportion of fat are often misdiagnosed as hepatocellular carcinoma or other liver tumours, such tumours lack immunoreactivity for keratin, hepatocytes, crystalline egg albumin and α‐fetoprotein, as shown in our patient.
Most AMLs are benign, but examples of malignant behaviour have been described for both renal and extrarenal AML. No clear criteria identify malignant tumours, but most reported examples have shown increased mitotic activity. In our patient, histological examination showed a benign picture, and the patient remains well without any evidence of recurrence after surgical resection.
In conclusion, the low fat content in hepatic AML easily causes diagnostic problems, regardless of imaging studies and pathology. Trace amounts of fat often cause radiologists to fail to take into account the possibility of hepatic AML preoperatively. Moreover, the predominant smooth‐muscle cell part in such cases also confuses pathologists. Thus, HMB45 seems to be a promising tool for establishing the correct diagnosis.
Abbreviations
AML - angiomyolipoma
HMB45 - homatropine methylbromide 45
PEC - perivascular epithelioid cell
SMA - smooth‐muscle actin
SMC - smooth‐muscle cell
Footnotes
Competing interests: None declared.
References
- 1.Hogemann D, Flemming P, Kreipe H.et al Correlation of MRI and CT findings with histopathology in hepatic angiomyolipoma. Eur Radiol 2001111389–1395. [DOI] [PubMed] [Google Scholar]
- 2.Sturtz C L, Dabbs D J. Angiomyolipomas: the nature and expression of the HMB45 antigen. Mod Pathol 19947842–845. [PubMed] [Google Scholar]
- 3.Bonetti F, Pea M, Martignoni G.et al Clear cell (“sugar”) tumor of the lung is a lesion strictly related to angiomyolipoma—the concept of a family of lesions characterized by the presence of the perivascular epithelioid cells (PEC). Pathology 199426230–236. [DOI] [PubMed] [Google Scholar]
- 4.Fadare O, Parkash V, Yilmaz Y.et al Perivascular epithelioid cell tumor (PEComa) of the uterine cervix associated with intraabdominal “PEComatosis”: a clinicopathological study with comparative genomic hybridization analysis. World J Surg Oncol 2004235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishak K G. Mesenchymal tumour of the liver. In: Okuda K, Peters RL, eds. Hepatocellular carcinoma. New York: Wiley, 1976247–307.
- 6.Tsui W M, Colombari R, Portmann B C.et al Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol 19992334–48. [DOI] [PubMed] [Google Scholar]
- 7.Chung A Y, Ng S B, Thng C H.et al Hepatic angiomyolipoma mimicking hepatocellular carcinoma. Asian J Surg 200225251–254. [DOI] [PubMed] [Google Scholar]
- 8.Sajima S, Kinoshita H, Okuda K.et al Angiomyolipoma of the liver—a case report and review of 48 cases reported in Japan. Kurume Med J 199946127–131. [DOI] [PubMed] [Google Scholar]
- 9.Terris B, Flejou J F, Picot R.et al Hepatic angiomyolipoma. A report of four cases with immunohistochemical and DNA‐flow cytometric studies. Arch Pathol Lab Med 199612068–72. [PubMed] [Google Scholar]
- 10.Goodman Z D, Ishak K G. Angiomyolipoma of the liver. Am J Surg Pathol 19848745–750. [DOI] [PubMed] [Google Scholar]
- 11.Nonomura A, Minato H, Kurumaya H. Angiomyolipoma predominantly composed of smooth muscle cells: problems in histological diagnosis. Histopathology 19983320–27. [DOI] [PubMed] [Google Scholar]
- 12.Svec A, Velenska Z. Renal epithelioid angiomyolipoma—a close mimic of renal cell carcinoma. Report of a case and review of the literature. Pathol Res Pract 2005200851–856. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi T, Itai Y, Takahashi M.et al Angiomyolipoma of the liver: significance of CT and MR dynamic study. Abdom Imaging 199823520–526. [DOI] [PubMed] [Google Scholar]
- 14.Gown A M, Vogel A M, Hoak D.et al Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol 1986123195–203. [PMC free article] [PubMed] [Google Scholar]
- 15.Barnard M, Lajoie G. Angiomyolipoma: immunohistochemical and ultrastructural study of 14 cases. Ultrastruct Pathol 20012521–29. [DOI] [PubMed] [Google Scholar]
- 16.Ren N, Qin L X, Tang Z Y.et al Diagnosis and treatment of hepatic angiomyolipoma in 26 cases. World J Gastroenterol 200391856–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong D R, Ji X L. Hepatic angiomyolipoma—misdiagnosis as hepatocellular carcinoma: a report of 14 cases. World J Gastroenterol 20006608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]