Abstract
The present studies were designed to develop a formulation of amphotericin B in a lipid-based preparation as a microemulsion and to compare its toxicity with the commercial formulation Fungizone®. The final product developed is a lyophilized amphotericin B, oil and surfactant blend for reconstitution in water to yield a microemulsion containing 5 mg/ml of the drug. Pseudoternary phase diagrams were constructed to identify areas of existence of microemulsion composed of Peceol® (glyceryl monooleate) as oil phase and Mys 40® (polyethylene glycol 40 stearate) and Solutol HS 15® (polyethylene glycol 15 hydroxy stearate) as surfactants. Amphotericin B was co-evaporated with oil - surfactant mixture to produce a microemulsion pre-concentrate. The co-evaporate was diluted in water, filtered for sterilization and lyophilized to obtain the final product. The lyophilized as well as the reconstituted products were separately studied for stability and the latter was also characterized for various physicochemical aspects including droplet size of the dispersed phase, osmolarity and aggregation state of drug. The dispersion showed no evidence of precipitation of drug for 48 h, and resisted destabilization due to freeze–thaw cycles or centrifugation. The dispersed phase globules measured a mean size of 84 nm and uv–spectrophotometric studies indicated the presence of self-aggregated amphotericin B. The present formulation showed a 92% decrease in haemolysis of human RBC in vitro when compared with the commercially available Fungizone®. The LD50 in mice was estimated to be 3.4 mg/kg. The results indicate that the formulation holds promise for development as a safer and efficacious alternative for amphotericin B therapy.
Key words: amphotericin B, microemulsion, Mys 40®, Peceol®, Solutol HS 15®
INTRODUCTION
Onset of the AIDS epidemic combined with the increased use of powerful immunosuppressive drugs for organ transplants and cancer chemotherapy has resulted in fungal diseases becoming an important public health concern. A 3-4-fold increase in mortality due to mycoses since the1980s is reported (1). Amphotericin B is the antifungal antibiotic of choice in the treatment of severe systemic fungal infections. Since its introduction in 1956, amphotericin B has remained the most effective systemic therapy for serious fungal infections such as candidiasis, histoplasmosis, and aspergillosis (2,3), and is the drug of choice for the treatment of disseminated mycosis in immuno-depressed patients. Also, even after 50 years of its clinical use, there are not many reports of fungal resistance to amphotericin B (4).
The clinical use of amphotericin B is however inconvenient due to
Need for parenteral administration: Due to poor absorption from gastrointestinal tract, intravenous route is the most preferred for effective systemic therapy. The drug is administered as an IV infusion since bolus doses cause severe toxic reactions.
Poor solubility: Its ring structure comprising of a hydrophobic heptane chain and a hydroxyl rich hydrophilic chain imparts an amphoteric nature to the molecule with the result that solubility of the drug is negligible in both aqueous and hydrophobic systems compared to the dose of the drug, which is 1 mg/kg. The conventional formulation of amphotericin B is a freeze-dried powder, which yields a micellar solution of amphotericin B with bile salt sodium desoxycholate on reconstitution (Fungizone® Bristol Myers-Squibb, Princeton, NJ).
Nephrotoxicity and hematological toxicity: The use of Fungizone® is associated with side effects such as fever and chills, bile rigors, nausea, hematological intolerance and considerable nephrotoxicity (4). The presentation of these side effects may be so severe that the dose may have to be reduced, or sometimes, therapy discontinued.
The latter two drawbacks mentioned above have been overcome to a significant extent by lipid based formulations such as Ambisome® (a liposomal preparation), Abelcet® (dispersion of ribbon like amphotericin B - lipid complex) and Amphocil® (colloidal dispersion of amphotericin B - cholesteryl sulphate complex) (5,6). The decrease in toxicity has been attributed to factors such as modified drug release, a different interaction with cellular membranes, dissimilar pharmacokinetics, or larger particle sizes. These formulations are however based on expensive technologies such as the preparation of liposomes and their clinical use results in a many fold increase in the cost of therapy (7).
In the present study an attempt was made to develop a simpler formulation for amphotericin B by delivering the drug in a lipid containing dosage form as in an oil-in-water microemulsion. Microemulsions are clear, transparent, optically isotropic and thermodynamically stable systems comprising of oil, water and surfactants. Because of their high solublization capacity, they can be used as carriers for injectable drugs having poor water solubility (8). We hypothesized that a microemulsion would be suitable medium for administration of a poorly soluble drug such as amphotericin B. In addition, presence of lipids in the formulation could help in reduction of toxicity without compromising on efficacy.
Incorporation of amphotericin B into such a system is a challenge since the drug has poor aqueous as well as oil solubility. Earlier reports on amphotericin B microemulsions are based on the use of alkaline pH to solubilize the drug (3,9). The present work examines the use of selected oil–surfactant combination, which when co-evaporated with drug, results in a microemulsion pre-concentrate. The pre-concentrate is readily miscible with water and yields a microemulsion containing the drug in solution.
MATERIALS AND METHODS
Materials
Amphotericin B was received as a gift sample from Bharat Serums and Vaccines Ltd., Mumbai, India. Mys 40® (Polyethylene glycol 40 stearate), Nikkol chemicals, Japan, Peceol® (Glyceryl mono-oleate), Gattesfosse and Solutol HS 15® (Polyethylene glycol 15 hydroxy stearate), BASF, Mumbai, India were obtained as gift samples. Acetonitrile and methanol (HPLC grade) and double distilled water (prepared in-house) were used for chromatographic studies. Dimethyl formamide and dextrose (analytical grade) were procured from S.D. fine chemicals, (Mumbai, India). Millex GV sterile filter units (0.22 μm) were purchased from Millipore.
Construction of Pseudoternary Phase Diagrams
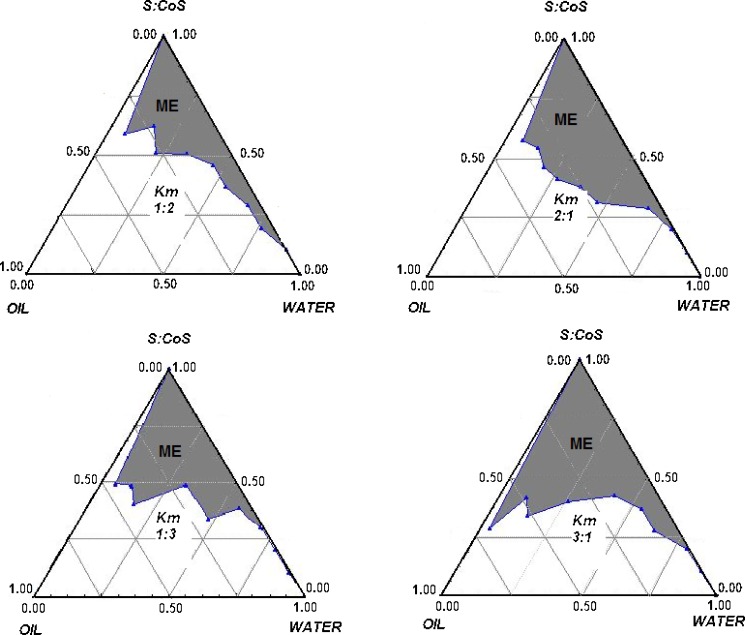
Pseudoternary phase diagrams were constructed for Peceol®-Mys 40®-Solutol HS 15® combinations at various Km ratios (surfactant/cosurfactant ratios) of Mys 40®/Solutol HS 15® such as 1:1, 1:2, 1:3, 2:1 and 3:1 to determine the area of microemulsion existence by an oil titration method. To a clear mixture of 2 g of water, surfactant and cosurfactant mixture in different ratios, oil was added in steady increments of 50 μl and the mixture was stirred using a glass rod until the addition of oil turned the mixture milky. Formation of o/w microemulsion was ascertained by examining transparent mixtures for miscibility by dilution with water and for optical isotropy by visualizing through a cross polarizer (fabricated using polarizing lens from Nikkon, Japan). No attempts were made to identify different phase systems and the phase diagram shows only the existence of microemulsion area.
Preparation of Amphotericin B Microemulsion (AmB-ME) Pre-concentrates
Weighed amounts of Peceol®/Mys 40® and Solutol HS 15® [1:6(2:1)] were warmed slightly and mixed together to form a homogeneous mixture. Drug (5 mg/gm of oil–surfactant mixture) was added and the drug–oil–surfactant mixture was dissolved in a minimum amount of dimethyl formamide. The solvent was then evaporated using a rotary vacuum evaporator (J-Sil, Mumbai) at a temperature of 70°C for 90 min. The resultant product was the AmB-ME pre-concentrate.
This pre-concentrate was dispersed in four times its weight of water and sterilized by filtration through a disposable syringe filter (0.22 μm Millipore filter) into 10 ml amber glass vials. Not more than 10 ml of microemulsion was filtered through each disposable syringe filter. The formulation was then frozen at −30°C for 12 h and dried with the application of vacuum for 24 h using a LABCONCO freeze on freeze dryer. The product was intended for reconstitution in dextrose injection (1 gm pre-concentrate/ml dextrose injection) to provide the AmB-ME with a strength equivalent to reconstituted Fungizone–5 mg Amphotericin B per milliliter.
Evaluation of Lyophilized Amphotericin B Microemulsion Pre-concentrate
The drug load in the product was determined in duplicate using a validated, sensitive HPLC method developed. Weighed quantity of AmB-ME pre-concentrate was dissolved in methanol and analyzed on μBondapack (25 cm, 5 μm) RP C-18 column using acetonitrile: EDTA (0.004 M) 75:25 as the mobile phase. The injection volume was 20 μl and detection was accomplished using a UV detector set at a wavelength of 405 nm (JASCO, Japan).
The pre-concentrate was reconstituted in 5% dextrose injection so as to obtain a drug concentration of 5 mg/ml. The pH of the resultant mixture was measured in duplicate at room temperature using a standardized pH meter (Universal Equipments, Mumbai). Osmolarity of samples diluted with dextrose injection to strength of 0.1 mg /ml of amphotericin B was determined in duplicate by the freezing point depression method using Osmomat 030 (Gonastec, Berlin, Germany).
A Beckman N5 plus submicron particle size analyzer was used to measure mean droplet size of the AmB-ME. Samples were prepared by dispersing 0.5 g of the AmB-ME pre-concentrate in 25 ml of dextrose injection. The solution was further diluted 100 fold with dextrose injection and analyzed for particle size. The dextrose injection used for dilution was prefiltered through 0.22 μm membrane.
Stress Testing
The AmB-ME pre-concentrate was dispersed in dextrose as described for the osmolarity determination, centrifuged at 5,000 rpm for 15 min, and observed for instability such as phase separation and change in particle size. The AmB-ME were subjected, in duplicate, to three freeze thaw cycles of 24 h each by freezing at a temperature of −20°C and thawing at a temperature of 32°C following which they were examined visually for phase separation and the globule size was also determined as described earlier.
Characterization of the Aggregation Form of Amphotericin B
The AmB-ME pre-concentrate was dispersed in 5% dextrose injection to yield a 4 μg/ml solution. The spectrum of this solution was recorded on a Shimadzu UV-160-A, spectrophotometer scanning the wavelength from 300 to 600 nm and was compared with a spectrum of a 4 μg/ml solution of pure Amphotericin B in methanol.
Stability Studies
The formulation under investigation includes the use of low melting excipients. Hence AmB-ME pre-concentrates prepared in the present study were refrigerated for storage. Stability on storage under the following conditions was assessed:
5°C ± 2°C for 2 months.
30°C ± 2°C/65% RH ± 5% RH (accelerated condition) for 2 months.
The product was packaged in amber glass vials. Samples were withdrawn at 0, 15, 30, and 60 day intervals from both the storage conditions. The freeze-dried product was reconstituted in dextrose as described earlier and assessed in duplicate for pH, particle size and drug content.
A linear regression plot of log (% amphotericin B residual content) against time was used to estimate the slope (m). The values for m were then substituted into the Arrhenius equation for the determination of the first order degradation rate constant (k):
 |
1 |
An estimate for the shelf life (the time for 10% loss, t90) was then calculated as:
 |
2 |
In Vitro Efficacy Studies
An agar cup plate diffusion method was used to evaluate the in vitro efficacy of the AmB-ME against fungi. Sabarouds agar seeded with a wild strain of Candida albicans was used to prepare plates. Five-millimeter holes were bored into the agar using a sterile borer. Two strengths of amphotericin B i.e. 5 μg/ml and 20 μg/ml were prepared for each of the following: AmB-ME diluted in dextrose injection, Fungizone® diluted in dextrose injection and a solution of amphotericin B in dimethylformamide. Of the solution, 70 μl was introduced into each cup using sterile micropipette. The solutions were randomly distributed across different plates so that each test solution was examined six times. The plates were then incubated at 25°C ± 0.2°C in a BOD chamber for 48 h. The possibility of dimethyl formamide and the oil–surfactant mixture producing inhibition of growth of the fungus was ruled out in preliminary studies. At the end of the incubation period, the sizes of zones of inhibition were estimated using a metric scale and the data was subjected to statistical analysis of variance.
In Vitro Hemolytic Studies
An in vitro test was used to assess the hemolytic potential of the developed injectable AmB-ME. Blood (10 ml) was withdrawn from a healthy human volunteer (who had rendered a signed writ consent) by vein puncture, into tubes containing 100 μl of heparin sodium injection. One part of this whole human blood was incubated with nine parts of various concentrations (5, 10, 15, 20 and 25 μg/ml) of Fungizone® or AmB-ME diluted in dextrose injection at a temperature of 37°C for 30 min. The tubes were then placed in a cold-water bath to arrest hemolysis. The samples were centrifuged at 2,500 rpm for 10 min and the clear supernatant was withdrawn, diluted appropriately with dextrose injection and an absorbance reading against blank (dextrose injection) was measured at a wavelength of 576 nm.
The % hemolysis was calculated as
 |
Where Abs, Abso and Abs100 are absorbance measurements of treated blood samples incubated with the test samples, dextrose injection (negative control) and 25 μg/ml of Fungizone® (positive control) respectively.
In Vivo Single Dose Acute Toxicity Study
The study was carried out as per OECD guideline 425 (up-and-down method) and was approved by the institutions ethical committee for animal studies. Swiss albino male mice were injected through the tail vein with various doses of AmB-ME formulations at an interval of 24 h. The first animal was dosed a step below the best estimate of the LD50 of Fungizone® i.e. 1.0 mg/kg as occurred from the literature (3). On the survival of the animal at that dose a second animal received a higher dose. The animals were observed for mortality or morbidity, critically during the first 30 min, and periodically for 24 h. Moribund animals were killed for humane reasons and considered as animals that died during the test as recommended in the test guidelines. Dosing was continued at fixed time interval of 24 h until four reversals occurred in five consecutive animals tested. The data obtained was fed into the “Acute Oral Toxicity (Guideline 425) Statistical Program” (AOT425StatPgm) and the LD50 was estimated.
RESULTS AND DISCUSSION
A preliminary solubility study not reported in this paper was used for selection of appropriate oil and surfactant, which would aid solubility of amphotericin B in the dosage form and allow for retaining the drug in solution even after dilution with water, since it is administered as an IV infusion. Reports of good solubility of amphotericin B in glyceryl mono-oleate (Peceol®) prompted inclusion of this oil in the study (10) Solubility in Peceol® determined at 30 ± 2°C was found to be 0.312 mg /ml. Further, amphotericin B is reported to form water soluble complexes with various PEG-derivatives including PEG 24 cholesterol, and PEG 100 stearate (11,12), and Pluronic F 127 & L 92 (13). Hence two PEG based surfactants were evaluated in the present study, polyethylene glycol 40 stearate (Mys 40®) and polyethylene glycol 15 hydroxy stearate (Solutol HS 15®), and were also found to form water soluble complexes with amphotericin B on co-evaporation from dimethyl formamide.
Figure 1(a–d) shows pseudoternary phase diagrams for Mys 40®–Solutol HS 15®–Peceol® systems at various Km ratios of 1:2, 1:3, 2:1 and 3:1. None of the transparent systems obtained showed liquid crystalline phases, and therefore, were said to be microemulsions. From the phase diagrams it was observed that area of microemulsion existence remained almost the same for a Km ratio of 1:2 or 2:1, however a Km ratio of 2:1 produced more transparent and stable systems. Km ratio of 3:1 produced the greatest area of microemulsion but most of this area consisted of w/o microemulsion as seen from their instability when diluted with water. A composition having a Mys 40®/Solutol HS 15® w/w ratio of 2:1 (57.14:28.57%) with 14.28% Peceol®, was selected for further studies.
Fig. 1.
Pseudoternary phase diagrams for Mys 40–Solutol–Peceol systems at various K m ratios
Earlier reports on the process of loading of amphotericin B into lecithin-based microemulsion have been based on dissolving drug in alkaline medium and incorporating into a preformed microemulsion at a temperature above the phase inversion temperature of the emulsifiers (3,9). In the present work we successively attempted a novel approach of co-evaporating drug with oil and surfactant mixture to generate AmB-ME pre-concentrates. Co-evaporation of drug–surfactant–oil mixture yielded a yellow opaque semisolid, which was readily dispersible in water. However, the diluted product showed separation of drug after 48 h at room temperature possibly due to slow dissociation of the drug–surfactant complex in presence of water. Hence it was concluded that the formulation should be prepared as the more stable pre-concentrate to be reconstituted by sterile dextrose solution (1 gm/1 ml) to yield the AmB-ME. To enable filtration sterilization, the co-evaporate was diluted with water, sterilized by filtration and stabilized further by lyophilization. Sterilization of the pre-concentrate by other means such as autoclaving was not found to be appropriate since it caused discoloration of the product and increase in droplet size of the microemulsion. Filtration has been successfully used for sterilization of microemulsion based injection (3,9).
The lyophilization process was conveniently carried out without the addition of any cryoprotectants and resulted in a waxy yellow product. The amphotericin content in this AmB-ME pre-concentrate was found to be 3.26 mg/g which was much lower than the theoretical content of 5 mg/gm. Loss of drug on filtration of amphotericin B formulations such as amphotericin B colloidal dispersion for parenteral use has been reported by Tipple et al. (14) and from amphotericin B microemulsion by Begona et al. (9). As reported in the study carried out by Tipple et al., a 0.1 mg/ml solution of amphotericin B in dextrose injection when filtered through 0.22 μ cellulose ester membrane showed a dramatic decrease in flow rate after the passage of as little as 30 ml of the solution. Flow ceased completely on passage of 100–200 ml solution with little or no drug present in the last amount filtered. Hence the 34.8% decrease in drug content observed in the present study was attributed to entrapment on the filter considering the colloidal nature of the dispersed phase. This observation was confirmed by analysis of drug content before and after filtration. Aseptic processing of presterilized ingredients is therefore possibly an appropriate method of preparing the developed injection.
The pH of AmB-ME having a concentration of 5 mg drug/ml in 5% dextrose injection at room temperature was found to be 4.2 ± 0.08 and the osmotic pressure was measured as 294.5 ± 1.5 mOsm/kg. The microemulsion was thus found to be isotonic. The average droplet size of AmB-ME diluted in dextrose injection is 83.8 ± 0.05 nm.
The AmB-ME obtained by dispersing the pre-concentrate in dextrose injection did not show any instability such as phase separation on subjection to centrifugation and freeze thaw cycles. Centrifugation also did not result in change of droplet size. Subjecting the product to freeze thaw cycles resulted in a decrease in droplet size as shown in Table 1.
Table 1.
Droplet Size of the Formulation on Stress Testing
| Test Condition | Droplet Size (nm)a |
|---|---|
| Before centrifugation | 83.8 ± 0.05 |
| After centrifugation | 83.4 ± 0.01 |
| Before freeze-thaw | 83.8 ± 0.03 |
| After freeze thaw | 38.7 ± 0.06 |
aData expressed as mean (n = 2)
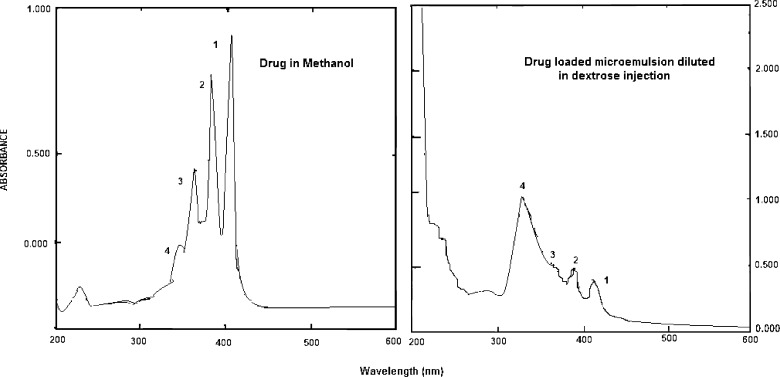
Amphotericin has within its molecule both hydrophilic and hydrophobic portions. Hence, in solution, at low concentrations it exists as a monomer but with increase in concentration, it shows various degrees of aggregation wherein the polyene chromophores are stacked together. The critical aggregation concentration of Amphotericin B is reported to be 1 μg/ml (15). Spectrophotometric studies are a useful aid in determining the state of aggregation of the drug since the forms show different absorption patterns. A UV spectrum of amphotericin B in an organic solvent such as methanol wherein the drug exists in monomeric form shows the presence of four absorption bands with decreasing intensities at 405 (1), 385 (2), 365 (3) and 344 (4) nm. As aggregation occurs and the self associated form is formed, the small band at 344 undergoes a blue shift to about 333 (4), increases in intensity and the other three bands undergo red shifts to about 368 (3), 390 (2) and 420 (1) respectively together with a reduction in intensity, indicating the appearance of the self aggregated form (16). A comparison of UV spectra of drug in methanol and AmB-ME diluted in dextrose is presented in (Fig. 2a and b). The changes in the spectrum of Amphotericin B clearly indicate the presence of the self -associated form of amphotericin B in the AmB-ME. Although the positions and intensities of the band may vary, the ratio of the intensities of absorption at the lowest (around 346): the highest (around 409) wavelength may be taken as a measure of the level of aggregation. This ratio is reported to be very low, (<0.25) for unaggregated or monomeric form and as high as 2.0 for highly aggregated form (17). The ratio of absorptivities at the lower and higher wavelengths for Amphotericin B in methanol and in the diluted AmB-ME were found to be 0.22 and 2.4 respectively, leading to the conclusion that the drug in microemulsion exists in aggregated form.
Fig. 2.
Study of aggregation state of amphotericin B/UV spectrum
A close association is believed to exist between state of aggregation of amphotericin B and its toxicity. The mechanism of action proposed for the lethal effect of amphotericin B on fungi is derived from its interaction with sterols in bilayer membranes such as cell walls causing either pore formation within the membrane, its complete destruction and/or inhibition of membrane repair. Monomeric amphotericin B associates with the sterols in fungal cell membranes, whereas self-associated amphotericin B can also form pores in cholesterol-containing membranes leading to toxicity towards host cells (18).
Amphotericin B preferably exists in monomeric form when complexed with Myrj 59® (polyethylene glycol 100 stearate) as reported by Tasset et al. (12). However in the present study amphotericin B is found to be in aggregated form despite complexation with Mys 40® (polyethylene glycol 40 stearate). This could be attributed to presence of Peceol® or its ratio with amphotericin B or the method of preparation. Self aggregation of the drug in presence of lipid is reported to depend on different factors, such as amphotericin B concentration, the drug: lipid ratio, the medium in which the drug is dispersed, action of surfactants, the way the dispersions are prepared and also the temperatures they have been exposed to (19).
The freeze-dried product showed no significant change in drug content, particle size and pH at refrigerated conditions (Table 2). There was also no apparent difference in physical characteristics such as color. The product had an amphotericin content of 95.17% of the initial content as determined by HPLC at the end of 2 months revealing a slow rate of degradation of drug on refrigeration. At the accelerated storage condition i.e. 30°C ± 2°C/65% RH ± 5% RH there was a significant decline in drug content. At the end of 2 months drug load in the product was found to be 87% of the initial content. However there was no change in the appearance, pH and particle size. The shelf life of the product estimated is 105 days on refrigeration, and 55 days on storage at 30°C ± 2°C/65% ± 5% RH (Table 3).
Table 2.
Results of Stability Studies of Freeze-Dried AmB-ME
| Characteristics | 0 days | 15 days | 30 days | 60 days | |||
|---|---|---|---|---|---|---|---|
| 5°C ± 2°C | 30°C ± 2°C /65% ± 5% RH | 5°C ± 2°C | 30°C ± 2°C /65 ± 5%RH | 5°C ± 2°C | 30°C ± 2°C /65 ± 5% RH | ||
| pH | 4.20 ± 0.08 | 4.25 ± 0.05 | 4.25 ± 0.03 | 4.20 ± 0. 03 | 4.27 ± 0.05 | 4.30 ± 0.04 | 4.32 ± 0.07 |
| Particle size (nm) | 83.7 ± 0.10 | 84.2 ± 0.15 | 84.2 ± 0.05 | 86.4 ± 0.10 | 88.1 ± 0.10 | 89.8 ± 0.25 | 92.1 ± 0.05 |
| Drug content (mg/g) | 3.26 ± 0.07 | 3.24 ± 0.02 | 2.92 ± 0.05 | 3.21 ± 0.02 | 2.80 ± 0.07 | 3.05 ± 0.03 | 2.36 ± 0.16 |
| Drug content (%) | 100.0 ± 0.06 | 99.5 ± 0.02 | 95.5 ± 0.05 | 99.0 ± 0.02 | 92.3 ± 0.07 | 95.2 ± 0.03 | 87.7 ± 0.16 |
Table 3.
Shelf Life and Degradation Rate of AmB-ME
| Storage condition | k (day−1) | t 90 (days) |
|---|---|---|
| 30°C ± 2oC/65%R.H. ± 5%R.H. | 1.89 × 10 | 03 55 |
| 5°C ± 2°C | 1.01 × 10 | 03 105 |
In vitro evaluation of antifungal activity revealed no significant difference between all the three treatment means indicating that the AmB-ME was as effective as Fungizone® or a solution of amphotericin B in dimethyl formamide against fungi in vitro.
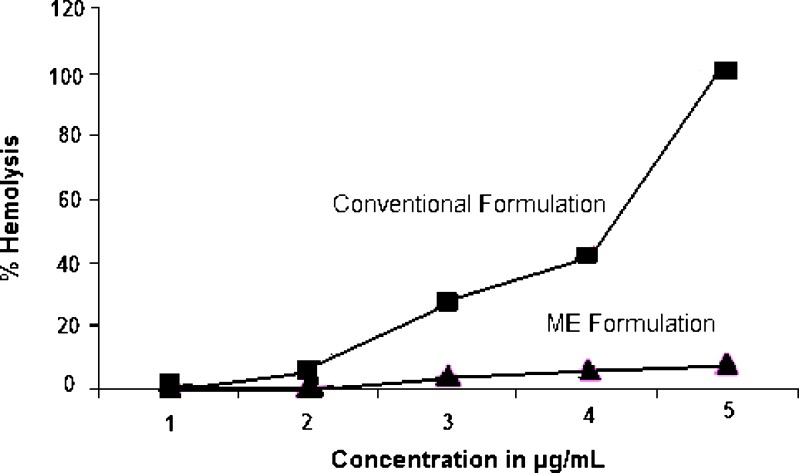
The use of amphotericin B (Fungizone®) is limited due to its hematological intolerance. The in vitro hemolytic activity of formulation when compared with that of Fungizone® on whole human blood revealed a significant reduction in drug induced hemolysis. This could be attributed to complexation of drug with PEG-derivatives. Tasset et al. (12) have reported a reduction in hemolytic activity of drug on complexation with PEG 100 stearate also brought about by co-evaporation from dimethylformamide, attributing the decrease to the protection offered to the cells by PEG 100 stearate. The authors have also cited the increased selectivity of the complexed amphotericin B for ergosterol to be another reason for decrease in hemolytic potential. In the present study amphotericin B also complexed with PEG 40 stearate and PEG hydroxy stearate 15 in presence of glyceryl mono-oleate revealed a 92.168% decrease in hemolytic activity in comparison with Fungizone® at the highest concentration examined, which, perhaps can be attributed to similar reasons considering the similar nature of the surfactants and the method of preparation (Fig. 3).
Fig. 3.
Hemolytic effect of amphotericin microemulsion on whole human blood in comparison with the conventional commercial formulation Fungizone®
The LD50 of the lyophilized microemulsion formulation was found to be 3.4 mg/kg signifying a decrease in toxicity over the conventional formulation Fungizone®. However it was less effective than alternate amphotericin B formulations in bringing about a reduction in toxicity. Similar observation of decrease in hemolytic potential with no significant change in the LD50 value has been reported for amphotericin B-PEG 100 stearate complex (12), and certain liposomal preparations of the drug (20). On the other hand a great deal of reduction in toxicity is reported with other lipid based amphotericin B formulations such as Ambisome® and Abelcet®. In our case some of the toxicity could be attributed to the presence of the drug in the aggregated form, which has greater toxicity to mammalian cells.
The LD50 values reported for commercialized (5) and investigated formulation of Amphotericin B (AMB) in mice are shown in Table 4.
Table 4.
LD50 Values of Different Amphotericin B Formulations in Mice (5) and of AmB-ME
| Product | Composition | LD50 (mg/kg) |
|---|---|---|
| Fungizone®® | Deoxycholate–AMB | 1.0 |
| AMB lyophilized microemulsion | Peceol®–Mys 40®–Solutol HS 15® AMB | 3.4 |
| Ambisome® | HSPC–Chol–DSPG–AMB | 175 |
| Abelcet® | DMPC–DMPG–AMB | 40 |
CONCLUSIONS
The study revealed the feasibility of formulating the poorly soluble molecule amphotericin B incorporated in a microemulsion. Microemulsion based delivery of amphotericin B investigated in this paper results in an equi-efficacious formulation but with lesser extent of some undesirable features of Fungizone® such as its hemolytic potential when examined in vitro. The study also reveals an improvement of LD50 value over the conventional amphotericin B formulation, indicating the possibility of development of the formulation for therapeutic use.
Acknowledgement
The authors wish to thank the All India Council for Technical Education, New Delhi, India for funding the project.
References
- 1.McNeil M., Stephanie L. N., Rana A. H., Maureen A. P., Laura A. C., Brian D. P., David W. W. Trends in mortality due to invasive mycotic diseases in the United States 1980–1997. Clin. Infect. Dis. 2001;33:641–647. doi: 10.1086/322606. [DOI] [PubMed] [Google Scholar]
- 2.Adedayo A., Bernardo J. F., Swenson C. E., Bolsack L. E., Horwith G., DeWit S., Kelly E., Klasterksy J., Sculier J. P., DeValeriola D., Anaissie E., Lopez-Berestein G., Llanos-Cuentas A., Boyle A., Branch R. A. Pharmacokinetic profile of ABELCET (amphotericin B lipid complex injection): combined experience from phase I and phase II studies. Antimicrob. Agents Chemother. 1997;41(10):2201–2208. doi: 10.1128/aac.41.10.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno M., Paloma F., Paloma B. Lyophilized lecithin based oil–water microemulsions as a new and low toxic delivery system for amphotericin B. Pharm. Res. 2001;18(3):344–351. doi: 10.1023/A:1011011215418. [DOI] [PubMed] [Google Scholar]
- 4.Brajtburg J., William G. P., George S. K., Gerald M. Amphotericin B: current understanding of mechanisms of action. Antimicrob. Agents Chemother. 1990;34(2):183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anya M. H. Supramolecular lipid drug delivery systems: from laboratory to clinic: a review of the recently introduced commercial liposomal and lipid based formulations of amphotericin B. Adv. Drug Deliv. Rev. 1997;24:345–363. doi: 10.1016/S0169-409X(96)00496-6. [DOI] [Google Scholar]
- 6.Ian M. H., Grant P. Lipid-based amphotericin B: a review of the last 10 years of use. Int. J. Antimicrob. Agents. 2001;17:161–169. doi: 10.1016/S0924-8579(00)00341-1. [DOI] [PubMed] [Google Scholar]
- 7.Wong-Beringer A., Jacobs R. A., Guglielmo B. J. Lipid formulations of amphoterecin B: clinical efficacy and toxicities. Clin. Infect. Dis. 1998;27:603–618. doi: 10.1086/514704. [DOI] [PubMed] [Google Scholar]
- 8.Jayne M., Gareth D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Delivery Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 9.Begona B., Marco A. M., Gloria F., Ballesteros M., Frutos P. Amphotericin B in oil–water lecithin-based microemulsions: formulation and toxicity evaluation. J. Pharm. Sci. 2002;91(4):1178–1185. doi: 10.1002/jps.10065. [DOI] [PubMed] [Google Scholar]
- 10.Verica R., Michael B., Eugene C., Kishor M. W. Effects of lipid-based oral formulations on plasma and tissue amphotericin B concentrations and renal toxicity in male rats. Antimicrob. Agents Chemother. 2003;47(10):3339–3342. doi: 10.1128/AAC.47.10.3339-3342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tasset C., Preat V., Roland M. The Influence of Myrj 59 on the solubility, toxicity and activity of amphotericin B. J. Pharm. Pharmacol. 1991;43:297–302. doi: 10.1111/j.2042-7158.1991.tb06693.x. [DOI] [PubMed] [Google Scholar]
- 12.Tasset C., Preat V., Bernard A., Roland M. Comparison of nephrotoxicities of different polyoxyethyleneglycol formulations of amphotericin B in rats. Antimicrob. Agents Chemother. 1992;36(7):1525–1531. doi: 10.1128/aac.36.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster D. Toxicity of solubilized and colloidal amphotericin B formulations to human erythrocytes. J. Pharm. Pharmacol. 1988;40:325–328. doi: 10.1111/j.2042-7158.1988.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 14.Tipple M., Shadomy S., Epsinel-Ingroff A. Availability of active amphotericin B after filtration through membrane filters. Am. Rev. Respir. Dis. 1977;115:879–881. doi: 10.1164/arrd.1977.115.5.879. [DOI] [PubMed] [Google Scholar]
- 15.Lavasanifar A., Samuel J., Sattari S., Kwon G. S. Block co-polymer micelles for the encapsulation and delivery of amphotericin B. Pharm Res. 2002;19:418–422. doi: 10.1023/A:1015127225021. [DOI] [PubMed] [Google Scholar]
- 16.Adams M. L., Kwon G. S. Relative aggregation state and hemolytic activity of amphotericin B encapsulated by poly(ethylene oxide)-block-poly (N-hexyl-l-aspartamide)acyl conjugate micelles: effects of acyl chain length. Biochim. Biophys. Acta. 1980;599:280–293. doi: 10.1016/0005-2736(80)90074-7. [DOI] [PubMed] [Google Scholar]
- 17.Kawabatta M., Maki O., Tomoyoshi M. Effect of aggregation of amphotericin B on lysophosphatidylcholine micelles as related to its complex formation with cholesterol or ergosterol. J. Biochem. 2001;129(5):725–732. doi: 10.1093/oxfordjournals.jbchem.a002912. [DOI] [PubMed] [Google Scholar]
- 18.Bolard J., Legrand P., Heitz F., Cybulska B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self association in the medium. Biochemistry. 1991;30:5707–5715. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Brunete J. A., Dea M. A., Rama S., Bolas F., Alunda J. M., Torrado-Santiagoa S., Torradoa J. J. Amphotericin B molecular organization as an essential factor to improve activity/toxicity ratio in the treatment of visceral leishmaniasis. J. Drug Target. 2004;00(0):1–8. doi: 10.1080/10611860400006596. [DOI] [PubMed] [Google Scholar]
- 20.Skoza F., Milholland D., Barza M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob. Agents. Chemother. 1987;31:421–429. doi: 10.1128/aac.31.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]