Abstract
Positive end-expiratory pressure (PEEP) protects the lung from injury during sustained ventilation, but its role in protecting the lung from injury during the initiation of ventilation in the delivery room is not established. We aimed to evaluate whether PEEP and/or tidal volume (VT) within the first 15-minutes of ventilation are protective against lung injury. Operatively delivered preterm lambs (133±1d gestation) were randomly assigned to unventilated controls or to 1 of four 15 minute ventilation interventions: 1) VT15mL/kg, PEEP 0cmH2O, 2) VT15mL/kg, PEEP 5cmH2O, 3) VT8mL/kg, PEEP 0cmH2O and 4) VT8mL/kg, PEEP 5cmH2O. Each group was subsequently ventilated with VT <10mL/kg, PEEP 5cmH2O for 1h 45min. Lung function was assessed and measurements of lung injury were evaluated post-mortem. After the 15min ventilation maneuver, the VT15 groups were hypocarbic, had higher oxygenation and required lower pressures then the VT8 groups; no consistent effect of PEEP was found. Markers of lung injury were significantly elevated in all ventilation groups compared to unventilated controls; no effect of PEEP was found. Ventilation resulted in localization of IL-6 to the small airways. Initial ventilation of preterm lambs with PEEP and/or VT of 8mL/kg did not prevent an inflammatory injury to the lung.
Keywords: Neonatal resuscitation, preterm, lung injury, inflammation, volutrauma
INTRODUCTION
The initiation of breathing at birth is an essential but complex adaptation that must rapidly transition the fluid filled fetal lung to gas exchange (1). Critical components of this transition are clearance of fluid from the airways, establishment of a functional residual capacity (FRC), and increased blood flow to the lungs. This transition often requires assistance for term infants and is more difficult and ineffective for preterm infants. A poor transition to air breathing in preterm infants is frequently due to surfactant deficiency, decreased respiratory drive, and perhaps more lung fluid as the majority of preterm infants are now delivered via cesarean section (2). Despite the frequent need for ventilatory assistance after delivery, and numerous experimental demonstrations that the preterm lung can be easily injured by mechanical ventilation (3, 4), there is minimal clinical information about how best to provide initial ventilatory assistance to the preterm lung. The equipment used for resuscitation of the preterm has not been standardized and does not allow for accurate control of tidal volume (VT), positive end-expiratory pressure/continuous positive airway pressure (PEEP/CPAP), or inspiratory times (5, 6).
Ventilation of the preterm lung for hours without PEEP will cause lung injury and high initial tidal volumes further increase that lung injury (3, 4, 7). A brief period of high VT ventilation without PEEP will injure the preterm lung and subsequent ventilation with PEEP will amplify that injury (8); this injury can be reduced by surfactant treatment prior to mechanical ventilation of the preterm lung (4, 9). In preterm sheep and baboons, CPAP decreases injury compared to mechanical ventilation without PEEP (10, 11). Ventilation of the fluid filled preterm lung has similarities to the lung injury resulting from ventilation of saline lavaged adult lungs, in that there is surfactant deficiency and fluid in the airways with atelectasis or fluid filled alveoli (12). Ventilation over-distends and injures the airways proximal to the atelectatic or fluid filled alveoli (13, 14). Atelectasis with non-uniform ventilation results in over-distension of the ventilated lung - the concept of the baby lung (15). Surfactant deficient fluid columns in small airways also cause fluid mechanical stresses that disrupt the airway epithelium (16). Volutrauma results from volumes that overstretch the lung regionally or from ventilation of collapsed lung units, and stretch induced injuries can occur in both the airways and the alveoli (12).
We examined how PEEP might moderate lung injury in the preterm lung to help develop recommendations for neonatal resuscitation. We hypothesized that initiation of ventilation in surfactant deficient preterm lambs using PEEP would decrease lung injury and that the injury caused by a large VT might be decreased with the use of PEEP, by minimizing collapse at end expiration. We evaluated volume-targeted ventilation using VT of 8 mL/kg or 15 mL/kg with and without PEEP for 15 min after birth and assessed lung injury 2 h after birth.
METHODS
Animal Handling and the Initial Ventilation Maneuver
Investigations were approved by the animal ethics committees of the Western Australian Department of Agriculture and Food, the University of Western Australia and Cincinnati Children’s Hospital Medical Center. Lambs were randomized prior to delivery to an unventilated control group, or to 1 of 4 ventilation regimes for the first 15 minutes: 1) VT 15mL/kg, PEEP of 0cmH2O, 2) VT 15mL/kg, PEEP of 5cmH2O, 3) VT 8mL/kg, PEEP of 0cmH2O, and 4) VT 8mL/kg, PEEP of 5 cmH2O. Preterm fetuses (133 ± 1 d gestational age; term 150 d gestational age) were exteriorised via cesearean section, a 4.5 mm endotracheal tube was inserted via a tracheotomy, and the assigned ventilation protocol was initiated immediately following delivery at 40 breaths/min using heated, humidified 40% oxygen in air and an inspiratory time of 0.7 s. Peak inspiratory pressures (PIP) were adjusted to achieve the target VT by 10 min of age and maintained at that VT until 15 min of age. Ventilated lambs were anaesthetized by infusion of Remifentanil (Domitor® 0.1mg/kg/min, Pfizer Animal Health, NSW, Australia) and Propofol (Repose®, 0.05 mg/kg/min, Norbrook Laboratories Ltd., Victoria, Australia). Control lambs (n=5) were euthanased immediately after delivery. Lambs did not receive corticosteroids or surfactant treatment.
Ventilation after 15 min
After 15 min of age, the 4 groups received ventilation designed to minimally injure the lung during the remaining ventilation period, using a respiratory rate of 40 breaths/min and 5cmH2O PEEP. Blood gas measurements every 30 min guided the ventilator adjustments. The combined targets for partial pressure of carbon dioxide in arterial blood (PaCO2) of 50-60mmHg and a VT <10 mL/kg were achieved by adjusting the PIP.
The fractional inspired oxygen (FiO2) was adjusted to target a partial pressure of oxygen in arterial blood (PaO2) of 40-100mmHg. Oxygenation index (OI) was calculated by the equation OI= mean airway pressurexFiO2x 100/PaO2. VT values were measured continuously with Florian Respiratory Monitors (Acutronic Medical Systems, Hirzel, Switzerland).
Lung Processing
At 2 h of age, animals were ventilated with FiO2 of 1 for 3min at 2h of age prior to clampint of the tracheal tube for 3 min to achieve atelactesis. The lambs received a lethal dose of pentobarbital (100mg/kg). After opening the chest, a deflation pressure-volume curve was constructed after gas inflation to 40cmH2O pressure (17). Bronchoalveolar lavage fluid (BALF) samples were obtained from saline lavage of the left lung (17).
Measurements on Lung Tissue
Protein (18), protein carbonyls (19), saturated phosphatidylcholine (20) and myeloperoxidase (21) were measured in BALF. Total RNA was isolated from the right lower lobe of the lung and liver, and 10 μg of total RNA was used for IL-1β, IL-6, IL-8, TNF-α, TGF-β1, and TLR-2 quantitation using RNAse protection assays (22). The right upper lobe of the lung was inflation fixed (Formalin) and in situ hybridization for IL-6 was performed (22).
Data Analysis and Statistics
Results are shown as mean (± SEM). Statistics were analyzed using SigmaStat 3.5 (Systat Software, Inc. San Jose, CA). For normally distributed data a one-way or two-way ANOVA with the Holm-Sidak multiple comparison procedure were used for comparisons between groups. A Kruskal-Wallis ANOVA on Ranks was used for non-normalized data. Significance was accepted as p<0.05.
RESULTS
Initial Ventilation Maneuver
The seven animals randomized to each group had similar body weights and cord blood gas values (Table 1). High cord blood PaCO2 values resulted from the anesthesia and positioning of the ewe for delivery. The tidal volumes achieved at 5, 10, and 15 min within the respective groups were similar with or without PEEP (Table 2). The use of PEEP also did not change the ventilatory pressures needed to achieve the VT except for the VT15 groups at 5 min and VT8 groups at 10 min. All animals required relatively high pressures to achieve the VT targets by 10 min of age. At 15 min, the VT15 groups were hypocarbic and the VT8 groups were hypercarbic. The oxygenation index was significantly lower in the VT15 groups compared to VT8 groups (p=0.012) irrespective of PEEP (Table 2). Therefore, there was no consistent effect of 5 cmH2O PEEP on ventilatory pressure, PaCO2 or Oxygenation Index at 15 min of age using volume targeted ventilation at either VT.
Table 1.
Description of Animals
| Nonventilated Controls | VT 15 | VT 8 | |||
|---|---|---|---|---|---|
| PEEP 0 | PEEP 5 | PEEP 0 | PEEP 5 | ||
| Number | 7 | 7 | 7 | 7 | 7 |
| Body Wt (kg) | 3.2 ± 0.2 | 3.5 ± 0.2 | 3.6 ± 0.3 | 3.4 ± 0.3 | 3.6 ± 0.7 |
| Cord Blood pH | - | 7.27 ± 0.02 | 7.20 ± 0.03 | 7.19 ± 0.04 | 7.21 ± 0.06 |
| Cord Blood PaCO2 (mmHg) | - | 61 ± 3 | 66 ± 3 | 69 ± 7 | 70 ± 7 |
| Saturated Phosphatidylcholine in BALF (μmol/kg) | 2.0 ± 0.6 | 3.8 ± 0.8 | 3.2 ± 0.8 | 2.1 ± 0.6 | 2.8 ± 0.6 |
Table 2.
Ventilation Maneuver after Delivery
| VT 15 | VT 8 | |||
|---|---|---|---|---|
| PEEP 0 | PEEP 5 | PEEP 0 | PEEP 5 | |
| VT (mL/kg) | ||||
| 5 min | 11.2 ± 0.9 | 10.2 ± 1.7 | 6.3 ± 0.7§ | 7.8 ± 1.4 |
| 10 min | 14.9 ± 0.6 | 14.0 ± 1.8 | 7.9 ± 0.4§ | 7.9 ± 0.8§ |
| 15 min | 16.4 ± 0.7 | 15.4 ± 1.5 | 7.9 ± 0.5§ | 8.4 ± 0.5§ |
| Ventilatory Pressures (cmH2O) | ||||
| 5 min | 36 ± 1 | 31 ± 1* | 31 ± 1 | 28 ± 1 |
| 10 min | 40 ± 2 | 37 ± 1 | 34 ± 1 | 28 ± 2* |
| 15 min | 42 ± 2 | 39 ± 2 | 32 ± 3 | 29 ± 2 |
| PaCO2 (mmHg) at 15 min | 25 ± 2 | 23 ± 2 | 71 ± 6§ | 60 ± 6§* |
| Oxygenation Index at 15 min | 7.6 ± 1.6 | 5.5 ± 0.5 | 15.0 ± 2.3§ | 10.6 ± 3.0§ |
Ventilatory pressures are peak inspiratory pressures minus positive end expiratory pressures (PEEP). Oxygenation Index = mean airway pressure x FiO2 x 100 / PaO2.
p<0.05 vs. same VT target group
p<0.05 vs VT15.
Maintenance ventilation to 2h of Age
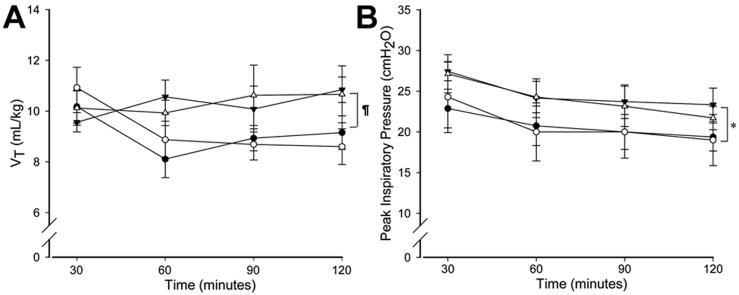
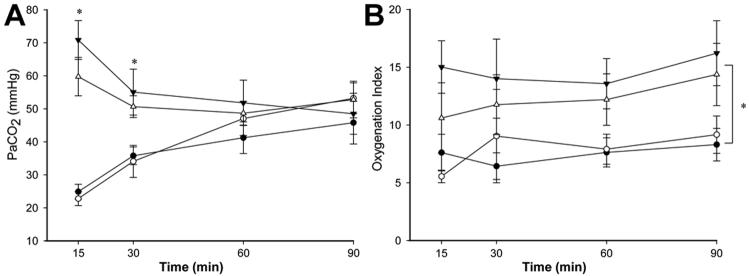
The ventilatory pressures required to maintain VT <10 mL/kg in the subsequent ventilation period were significantly higher for the VT8 groups compared to VT15 groups (p=0.006) irrespective of PEEP (Fig. 1). However, VT was higher in VT8 groups for the remainder of the study compared to VT15 groups (p=0.067; Fig. 1). PaCO2 values were not different between groups after 30 min of age (Fig. 2). In contrast, OI were lower for the groups receiving the initial VT15 ventilation maneuver relative to the VT8 groups (p<0.001), irrespective of PEEP (Fig. 2). Therefore, the use of PEEP had no consistent effect on lung physiology over 2h. The initial 15 min of VT15 ventilation improved lung function as measured by ventilatory pressures and oxygenation over the entire subsequent period of ventilation.
Figure 1.
PaCO2 (A) and Oxygenation Index (OI), B) in ventilation groups following the 15 min initial ventilation period up to 90 min. Values at 120 min are not included because the lambs were ventilated with 100% oxygen to facilitate lung collapse. ▼; VT 8 PEEP 0, △: VT 8 PEEP 5, ●: VT 15 PEEP 5, ○: VT 15 PEEP 0. Groups receiving VT of 15 mL/kg during initial ventilation had lower PaCO2 prior to normalization after 30 min, and a lower oxygenation index throughout subsequent ventilation, compared to groups that received VT of 8 mL/kg. Group means ± SEM shown. * p<0.05 VT15 mL/kg vs. VT8 mL/kg. ¶ p = 0.067 VT15 mL/kg vs. VT8 mL/kg.
Figure 2.
Tidal volume VT (A) and Peak inspiratory pressure (B) in ventilatory groups after the initial ventilation period. ▼: VT 8 PEEP 0, △: VT 8 PEEP 5, ●: VT 15 PEEP 5, ○: VT 15 PEEP 0. * p<0.05 VT 15 mL/kg vs. VT 8 mL/kg. Groups receiving high VT during the initial 15 min ventilation period required lower ventilatory pressures to maintain a VT of 8 mL/kg during subsequent ventilation.
Indicators of Injury
Deflation pressure-volume curves were similar for the ventilated groups: lung volumes measured at 40 cmH2O were about 50 mL/kg (Fig. 3). The amounts of surfactant in the BALF were similar for the ventilation groups (Table 1). BALF from ventilated lungs contained more protein than did the BALF of the unventilated lungs reflecting an epithelial permeability abnormality (Fig. 4). There were no differences between the ventilated groups and no significant protection with PEEP (p=0.088). Expression of the pro-inflammatory cytokine mRNA IL-1β, IL-6, and IL-8 was greatly elevated in all ventilation groups compared to unventilated controls, with no differences between the ventilation groups (Fig. 4). Protein Carbonyls, a measure of oxidative injury, and TLR-2 mRNA were higher in all ventilation groups relative to unventilated controls. (Table 3). Myeloperoxidase (MPO), a marker of neutrophil activity, was not different between ventilation groups and unventilated controls. TNFα and TGF-β1 mRNA in the lungs were similar between all groups (Table 3). IL-6 was expressed highly in terminal bronchiolar and alveolar duct epithelium of the ventilated lambs, with sparing of large bronchi (Fig. 5). The pattern and quantity of expression was similar in all ventilated groups.
Figure 3.
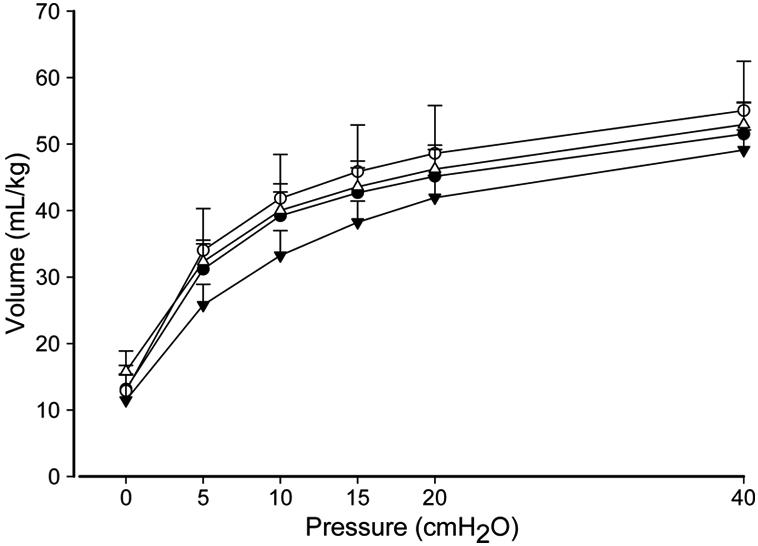
Deflation pressure-volume curves. ▼: VT 8 PEEP 0, △: VT 8 PEEP 5, ●: VT 15 PEEP 5, ○: VT 15 PEEP 0. Volumes at 15, 20 and 40 cmH2O were similar between ventilation groups. Group means ± SEM shown.
Figure 4.
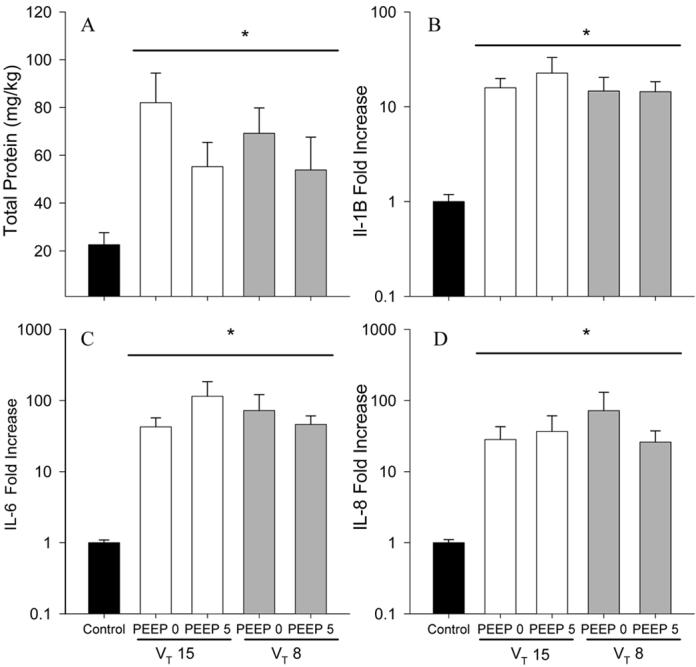
Total protein (A), IL-1β (B) IL-6 (C) and IL-8 (D) mRNA from lung tissue in lambs receiving VT 15 mL/kg (□) or VT 8 mL/kg ( ) receiving either 0 cmH2O or 5 cmH2O PEEP during the initial resuscitation period; expressed as fold increase relative to control (non-ventilated) lambs (■). Group means ± SEM shown. *p<0.05 vs. controls.
) receiving either 0 cmH2O or 5 cmH2O PEEP during the initial resuscitation period; expressed as fold increase relative to control (non-ventilated) lambs (■). Group means ± SEM shown. *p<0.05 vs. controls.
Table 3.
Assessment of Injury/Inflammation
| Nonventilated Controls | VT 15 | VT 8 | |||
|---|---|---|---|---|---|
| PEEP 0 | PEEP 5 | PEEP 0 | PEEP 5 | ||
| TNF-α (mRNA fold increase) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.8 ± 0.4 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| TGF-β1 (mRNA fold increase) | 1.0 ± 0.02 | 1.1 ± 0.03 | 1.1 ± 0.02 | 1.0 ± 0.05 | 1.0 ± 0.07 |
| TLR2 (mRNA fold increase) | 1.0 ± 0.1 | 2.7 ± 0.3* | 3.1 ± 1.0* | 2.8 ± 0.9* | 2.3 ± 0.4* |
| Protein Carbonyl (nmol/mg) | 0.06 ± 0.02 | 0.42 ± 0.02* | 0.38 ± 0.07* | 0.38 ± 0.20* | 0.29 ± 0.09* |
| Myeloperoxidase (nmol/g) | 2.3 ± 0.6 | 3.7 ± 1.1 | 3.0 ± 0.8 | 5.0 ± 2.4 | 2.4 ± 0.6 |
p<0.05 vs. Nonventilated controls
Figure 5.
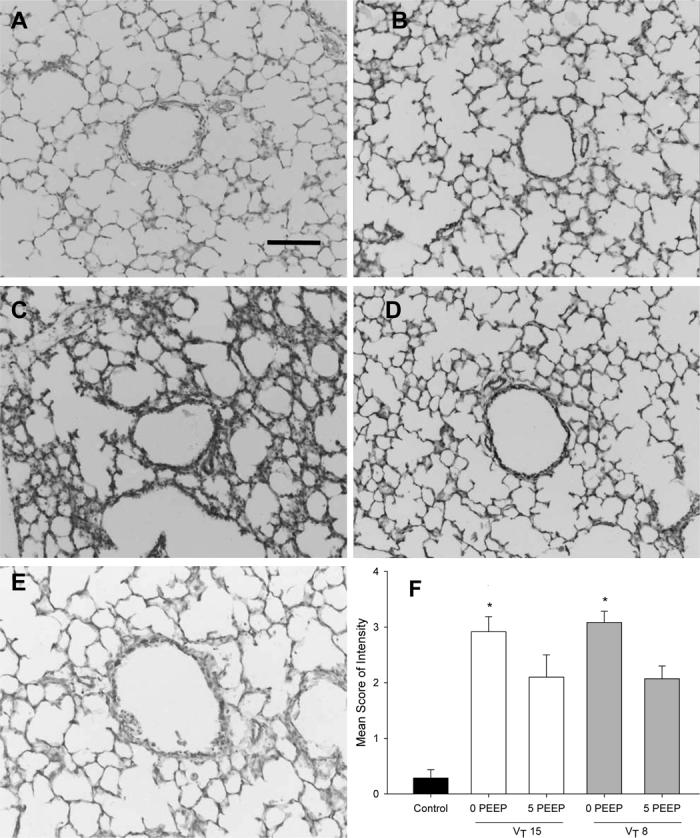
Localisation of IL-6 mRNA expression in control (A-B), a lamb ventilated with VT 15 mL/kg, PEEP 0 cmH2O (C), and a lamb ventilated with VT 8 mL/kg, PEEP 5 cmH2O (D). IL-6 expression is depicted in black silver grains. No IL-6 expression was detected in control lambs (n=2-3/group). Mechanical ventilation robustly induced IL-6 expression in the terminal bronchiolar/alveolar duct epithelium (bold arrows; see panel C), inflammatory cells (open arrowhead) (panels C&D) and bronchial smooth muscle (dark arrowhead; see panel D). Note the absence of IL-6 expression in the large bronchial epithelium (open arrow in panel D). (Bar denotes 50μm, Br=Bronchus). F) Qualitative assessment of mean score of intensity of IL-6 expression. * p<0.05; 5 cmH2O PEEP vs 0 cmH2O PEEP. All ventilation groups have significantly more IL-6 staining than unventilated controls.
Histology for Lung Injury
The tissue from the ventilated lungs showed minimal inflammation, some atelectasis and over-distended regions without bleeding or remarkable airway injury. There were no differences in histology between groups, and no evidence of severe lung injury.
DISCUSSION
Recent Cochrane Reviews concluded that there is insufficient evidence to recommend CPAP/PEEP for resuscitation in term or preterm infants because there were no studies that were adequate for review (23, 24). Our studies were designed to represent a controlled version of what occurs during resuscitation of the preterm with the variables being VT and PEEP. In practice, 76% of neonatology units provide CPAP or PEEP during resuscitation, and the preferred pressure level is 5 cmH2O (25). The 15 min resuscitation interval approximates the time required for neonatal resuscitations (6). We anticipated that the VT15, PEEP 0 group would have lung injury and investigated if a PEEP of 5 cmH2O might minimize that injury. The 15 mL/kg volume was chosen because many infants receive large VT inadvertently and this volume was used previously in preterm lamb models to cause severe lung injury (4, 8). The group given VT8, PEEP 5 was selected to represent an optimal initial ventilation as clinicians most frequently use 5 cmH2O PEEP (25), and 8 mL/kg is the normal tidal volume for spontaneously breathing preterm lambs on CPAP (26). We hypothesized that the recruitment of a VT of 8 mL/kg over the first 10 min with the use of 5 cmH2O PEEP would be less-injurious compared to the other groups. However, all groups had similar increases in BALF protein, lung tissue cytokine expression and the other biochemical indicators of injury. The conclusion is that neither the VT of 8 mL/kg nor the use of PEEP protected these preterm lungs.
The amount of lung injury during the initiation of ventilation depends on variables that differ with each experimental design. Surfactant treatment prior to the use of similar VT and PEEP did protect the lungs (4, 9). Probyn, et al., found that initiation of ventilation in more preterm lambs (125 d) required high PIP and PEEP improved oxygenation, although they did not evaluate lung injury (27). VT of 15 mL/kg, PEEP 0 for 15 min was evaluated in 128 d gestation lambs either returned to the intrauterine environment or delivered, surfactant treated, and ventilated for 3 h (8); all groups had lung injury, but ventilation following the injury greatly amplified the expression of IL-6 and IL-8 in the lungs. Thus, the injury caused by the initial ventilation maneuver was masked, in part, by the subsequent ventilation. Other studies compared spontaneously breathing lambs at 130-134 d gestation given CPAP with lambs ventilated comparably to the VT 8, PEEP 5 group, and neither group had large increases in biochemical markers of lung injury (10). The caveat to those studies was treatment of the ewes with betamethasone and epostane (a progesterone antagonist) to induce spontaneous breathing in the lambs at delivery. In the ventilated newborn mouse, alterations in lung architecture and septation can occur without overt inflammation (28). The injury response is developmental stage specific and may differ by species studied.
We interpret our results within the context of the effects of VT on the fluid filled surfactant-deficient lung. We chose animals at 133 d gestation because we wanted animals with surfactant deficiency that could be ventilated without severe lung injury. Small surfactant pool sizes, total lung volumes of about 50 mL/kg and ventilation pressures at 2h of age of about 20 cmH2O pressure all indicate lung immaturity. Of interest, the animals initially ventilated with higher VT of 15 mL/kg required less pressure and had better oxygenation over the subsequent 1 h 45 min than the VT 8 mL/kg animals. This suggests that these preterm lungs needed a high VT to “open up” or recruit the lung. The initial recruitment did not result in more injury in our animals.
Surfactant has important effects on the fetal lung during transition to air breathing. The term fetal lung secretes surfactant into the fetal lung fluid and concurrently absorbs fetal lung fluid during the terminal phases of delivery (29). As surfactant pool sizes are 10-20 fold higher at term than at any other time during life, the concentration of surfactant in the residual fetal lung fluid is high (30). Surfactant facilitates clearance of fluid from the airways and strikingly lowers the opening pressures of the lung (31). Fluid movement in small airways can injure the epithelium by fluid mechanical stress (16). The fetuses we studied did not experience labor, were surfactant deficient, and had fluid removed only from the large airways prior to ventilation. The high PIP of up to 40-50 cmH2O for the first 15 min were needed to clear the airways of fluid. Ventilation without surfactant results in over distention of the open lung because of the non-uniform surface tensions throughout the lung (32).
Therefore, the likely sequence that resulted in large increases in multiple indicators of lung injury in this experiment is as follows: The initial breaths given at about 35 cmH2O pressure using a volume targeted strategy delivered VT of 6-8 mL/kg at 5 min primarily to the airways. The FRC of the airways may have been maintained with the PEEP of 5 cmH2O. Each VT then over distended the compliant preterm airways resulting in the airway injury indicated by expression of IL-6 in the small airways and bronchi. As the lungs progressively opened, the more compliant open lung regions became over-distended. At autopsy, some lung parenchyma was not inflated with a static pressure of 40 cmH2O. This sequence of lung injury has been described with ventilation of the saline-lavaged adult lung (12-14).
The beneficial effects of PEEP may not be as evident in the transition of the fluid-filled lung to air breathing as in the aerated lung. PEEP can improve oxygenation (27, 33), but it did not over the first 15 min in our study. The small airways may not collapse because of foam or fluid pockets in the small airways which can splint the lung during the transition to air breathing (34), while surfactant deficient fluid may promote lung injury (16). Perhaps a higher PEEP would have decreased injury and improved oxygenation in this trial and in surfactant deficient lungs. A recent study by us showed that conventional mechanical ventilation with 10 cmH2O PEEP resulted in increased lung injury (Schulzke et al. Submitted). However, no indications were found that PEEP decreased injury during the transition to air breathing. Indeed, IL-6 expression in the bronchi and alveolar epithelium was not different between ventilated groups.
In clinical practice, VT is an unregulated and non-measured variable in the delivery room. VT is likely to be large and cause hyperventilation (6). Some delivery rooms use ventilators to control VT and pressures, and VT targeted ventilation is thought to be ideal for ventilation of infants to minimize the risk of BPD (35). Our results demonstrate the risks of VT targeted ventilation of the fluid filled lung. These lungs required high peak inspiratory pressures to deliver a VT of 8 mL/kg to lungs with total lung capacities of 50 mL/kg. Furthermore, using a rate of 40 breaths/min and a long inspiratory time to achieve the VT, the lambs were hypercarbic with PaCO2 values of 60-70 mmHg at 15 min of age. These results suggest that lung injury will be the inevitable consequence of attempts to achieve normocarbia quickly in a surfactant deficient and fluid-filled lung.
The lack of PEEP and the use of VT15 as the target volume for the first 15 min did not consistently increase indices of injury at 2 h. The assessment at 2 h is very early in the progression of lung injury, but the high expression of IL-1β and IL-8 mRNA and the increase in protein carbonyls suggest that more inflammation would be apparent later. The lack of differences between the experimental groups could result from a stretch injury, with the VT8 being sufficient to initiate a maximum response that was replicated by the other groups. Also, the need for subsequent ventilation may have amplified the initial injuries and masked any differences (8). Other variables of ventilation, such as the inspiratory flow and achievement of plateau pressure have not been adequately studied. Also oxygen exposure may also contribute to the injury although we minimized oxygen exposure for these experiments. There could be substantial differences for injury markers that we did not measure. Our experimental conditions were controlled relative to the human experience and thus the translation of these observations to the preterm infant must be cautious. Very low birthweight preterm infants deliver because of an abnormality with the pregnancy, most receive some antenatal corticosteroid exposure, and many are exposed to some preterm labor. Nevertheless, the frequent use of Cesarean section assures that many of these infants have large amounts of fluid in their lungs. These studies provide a clear example of how VT targeted ventilation can injure the preterm lung during the initiation of ventilation.
ACKNOWLEDGEMENTS
We gratefully acknowledge the technical assistance of Ms Jennifer Henderson, Dr Sven Schulzke, Dr Belinda Joyce and Amy Whitescarver.
STATEMENT OF FINANCIAL SUPPORT
This research was supported by grant NH-12714 from NICHD, Women and Infants Research Foundation, Fisher & Paykel Healthcare, a NH&MRC Career Development Award (J.J.P.: 404102) and a RD Wright NH&MRC Postdoctoral Fellowship (T.J.M.M.: 303261). Currently, G.R.P. is supported by a NHMRC and NHFA Fellowship and J.J.P. by a Sylvia and Charles Viertel Senior Medical Research Fellowship.
OTHER FINANCIAL SUPPORT
CPAP circuits, humidifiers and radiant warmer beds were provided by Fisher & Paykel Healthcare™(NZ).
Abbreviations
- BALF
Bronchoalveolar lavage fluid
- BPD
Bronchopulmonary dysplasia
- CPAP
Continuous positive airway pressure
- FRC
Functional residual capacity
- PEEP
Positive end-expiratory pressure
- PIP
Peak inspiratory pressure
- VT
Tidal volume
REFERENCES
- 1.Hooper SB, Kitchen MJ, Wallace MJ, Yagi N, Uesugi K, Morgan MJ, Hall C, Siu KK, Williams IM, Siew M, Irvine SC, Pavlov K, Lewis RA. Imaging lung aeration and lung liquid clearance at birth. FASEB J. 2007;21:3329–3337. doi: 10.1096/fj.07-8208com. [DOI] [PubMed] [Google Scholar]
- 2.Högberg U, Holmgren PA. Infant mortality of very preterm infants by mode of delivery, institutional policies and maternal diagnosis. Acta Obstet Gynecol Scand. 2007;86:693–697. doi: 10.1080/00016340701371306. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, Vilstrup CT. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res. 1997;42:348–355. doi: 10.1203/00006450-199709000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Wada K, Jobe AH, Ikegami M. Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol. 1997;83:1054–1061. doi: 10.1152/jappl.1997.83.4.1054. [DOI] [PubMed] [Google Scholar]
- 5.The International Liaison Committee on Resuscitation The International Liaison Committee on Resuscitation (ILCOR) consensus on science with treatment recommendations for pediatric and neonatal patients: neonatal resuscitation. Pediatrics. 2006;117:e978–e988. doi: 10.1542/peds.2006-0350. [DOI] [PubMed] [Google Scholar]
- 6.Stenson BJ, Boyle DW, Szyld EG. Initial ventilation strategies during newborn resuscitation. Clin Perinatol. 2006;33:65–82. doi: 10.1016/j.clp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Naik AS, Kallapur SG, Bachurski CJ, Jobe AH, Michna J, Kramer BW, Ikegami M. Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am J Respir Crit Care Med. 2001;164:494–498. doi: 10.1164/ajrccm.164.3.2010127. [DOI] [PubMed] [Google Scholar]
- 8.Hillman NH, Moss TJ, Kallapur SG, Bachurski CJ, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–581. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingimarsson J, Bjorklund LJ, Curstedt L, Gudmundsson S, Larsson A, Robertson B, Werner O. Incomplete protection by prophylactic surfactant against the adverse effects of large lung inflations at birth in immature lambs. Intensive Care Med. 2004;30:1446–1453. doi: 10.1007/s00134-004-2227-3. [DOI] [PubMed] [Google Scholar]
- 10.Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatr Res. 2002;52:387–392. doi: 10.1203/00006450-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Thomson MA, Yoder BA, Winter VT, Martin H, Catland D, Siler-Khodr T, Coalson JJ. Treatment of immature baboons for 28 days with early nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2004;169:1054–1062. doi: 10.1164/rccm.200309-1276OC. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchida S, Engelberts D, Roth M, McKerlie C, Post M, Kavanagh BP. Continuous positive airway pressure causes lung injury in a model of sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L554–L564. doi: 10.1152/ajplung.00143.2005. [DOI] [PubMed] [Google Scholar]
- 13.Bellardine Black CL, Hoffman AM, Tsai LW, Ingenito EP, Suki B, Kaczka DW, Simon BA, Lutchen KR. Relationship between dynamic respiratory mechanics and disease heterogeneity in sheep lavage injury. Crit Care Med. 2007;35:870–878. doi: 10.1097/01.CCM.0000257331.42485.94. [DOI] [PubMed] [Google Scholar]
- 14.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 15.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31:776–784. doi: 10.1007/s00134-005-2627-z. [DOI] [PubMed] [Google Scholar]
- 16.Huh D, Fujioka H, Tung Y-C, Futai N, Paine R, Grotberg JB, Takayama S. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci USA. 2007;104:18886–18891. doi: 10.1073/pnas.0610868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobe AH, Newnham JP, Willet KE, Moss TJ, Gore Ervin M, Padbury JF, Sly P, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med. 2000;162:1656–1661. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. Erratum in: Free Radic Biol Med 24(7-8):1352. [DOI] [PubMed] [Google Scholar]
- 20.Mason RJ, Nellenbogen J, Clements JA. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976;17:281–284. [PubMed] [Google Scholar]
- 21.Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- 22.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L527–L536. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- 23.Subramaniam P, Henderson-Smart DJ, Davis PG. Prophylactic nasal continuous positive airways pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD001243.pub2. CD001243. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell C, Davis PG, Morley CJ. Positive end-expiratory pressure for resuscitation of newborn infants at birth. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004341.pub2. CD004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leone TA, Rich W, Finer NN. A survey of delivery room resuscitation practices in the United States. Pediatrics. 2006;117:e164–e175. doi: 10.1542/peds.2005-0936. [DOI] [PubMed] [Google Scholar]
- 26.Mulrooney N, Champion Z, Moss TJ, Nitsos I, Ikegami M, Jobe AH. Surfactant and physiologic responses of preterm lambs to continuous positive airway pressure. Am J Respir Crit Care Med. 2005;171:488–493. doi: 10.1164/rccm.200406-774OC. [DOI] [PubMed] [Google Scholar]
- 27.Probyn ME, Hooper SB, Dargaville PA, McCallion N, Harding R, Morley CJ. Effects of tidal volume and positive end-expiratory pressure during resuscitation of very premature lambs. Acta Paediatr. 2005;94:1764–1770. doi: 10.1111/j.1651-2227.2005.tb01851.x. [DOI] [PubMed] [Google Scholar]
- 28.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice.: Prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol. 2008;294:L3–L14. doi: 10.1152/ajplung.00362.2007. [DOI] [PubMed] [Google Scholar]
- 29.Carlton DP, Cho SC, Davis P, Lont M, Bland RD. Surfactant treatment at birth reduces lung vascular injury and edema in preterm lambs. Pediatr Res. 1995;37:265–270. doi: 10.1203/00006450-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Rebello CM, Jobe AH, Eisele JW, Ikegami M. Alveolar and tissue surfactant pool sizes in humans. Am J Respir Crit Care Med. 1996;154:625–628. doi: 10.1164/ajrccm.154.3.8810596. [DOI] [PubMed] [Google Scholar]
- 31.Rider ED, Ikegami M, Whitsett JA, Hull W, Absolom D, Jobe AH. Treatment responses to surfactants containing natural surfactant proteins in preterm rabbits. Am Rev Respir Dis. 1993;147:669–676. doi: 10.1164/ajrccm/147.3.669. [DOI] [PubMed] [Google Scholar]
- 32.Pinkerton KE, Ikegami M, Dillard LM, Jobe AH. Surfactant treatment effects on lung structure and type II cells of preterm ventilated lambs. Biol Neonate. 2000;77:243–252. doi: 10.1159/000014223. [DOI] [PubMed] [Google Scholar]
- 33.Polglase GR, Morley CJ, Crossley KJ, Dargaville PA, Harding R, Morgan DL, Hooper SB. Positive end-expiratory pressure differentially alters pulmonary hemodynamics and oxygenation in ventilated, very premature lambs. J Appl Physiol. 2005;99:1453–1461. doi: 10.1152/japplphysiol.00055.2005. [DOI] [PubMed] [Google Scholar]
- 34.Scarpelli EM. Physiology of the alveolar surface network. Comp Biochem Physiol A Mol Integr Physiol. 2003;135:39–104. doi: 10.1016/s1095-6433(02)00352-5. [DOI] [PubMed] [Google Scholar]
- 35.Lindner W, Högel J, Pohlandt F. Sustained pressure - controlled inflation or intermittent mandatory ventilation in preterm infants in the delivery room? A randomized, controlled trial on initial respiratory support via nasopharyngeal tube. Acta Paediatr. 2005;94:303–309. doi: 10.1111/j.1651-2227.2005.tb18431.x. [DOI] [PubMed] [Google Scholar]