Abstract
Background
Patients with cystic fibrosis (CF) are at risk for early bone loss, and demonstrate increased risks for vertebral fractures and kyphosis. A multicenter, randomized, controlled trial was conducted to assess the efficacy, tolerability, and safety of therapy with oral alendronate (FOSAMAX; Merck; Whitehouse Station, NJ) in adults with CF and low bone mass.
Methods
Participants received placebo or alendronate, 70 mg once weekly, for 12 months. All participants received 800 IV of vitamin D and 1,000 mg of calcium daily. Adults with confirmed CF with a bone mineral density (BMD) T score of < − 1.0 were eligible for inclusion. Participants who had undergone organ transplantation or had other reported contraindications were excluded from the study. The primary outcome measure was the mean (± SD) percentage change in lumbar spine BMD after 12 months. Secondary measures included the percentage change in total hip BMD, the number of new vertebral fractures (grade 1 or 2), and changes in quality of life.
Results
A total of56 participants were enrolled in the study (mean age, 29.1 ± 8.78 years; 61%male). The absolute percentage changes in lumbar spine and total hip BMDs at follow-up were significantly higher in the alendronate therapy group (5.20 ± 3.67% and 2.14 ± 3.32%, respectively) than those in the control group (− 0.08 ± 3.93% and − 1.3 ± 2.70%, respectively; p < 0.001). At follow-up, two participants (both in the control group) had a new vertebral fracture (not significant), and there were no differences in quality of life or the number of adverse events (including serious and GI-related events).
Conclusion
Alendronate therapy was well tolerated and produced a significantly greater increase in BMD over 12 months compared with placebo.
Trial registration
ClinicalTrials.gov Identifier: NCT00157690
Keywords: alendronate, cystic fibrosis, low bone mass, osteoporosis, randomized controlled trial
The life expectancy for patients with cystic fibrosis (CF) has increased Significantly in the past several decades.1 As a result, long-term sequelae of the disease are becoming apparent in late adolescence and into adulthood. Low bone mass is common in CF patients and has been termed CF-related bone disease.2 The clinical manifestations of CF-related bone disease include an increased risk of fracture and kyphosis,3 with the potential consequence of an accelerated decline in lung function. 2,4,5 These physical manifestations may present a contraindication to lung transplantation, which is an important treatment option for many CF patients.6,7
The mechanism for early bone loss and fractures in CF patients is multifactorial and is likely due to several CF-related factors that also influence bone metabolism.2,3 These include delayed pubertal maturation, the malabsorption of vitamin D, poor nutritional status, inactivity, hypogonadism, and the frequent use of glucocorticoid therapy. Another potential mechanism is that the chronic pulmonary inflammation associated with CF leads to elevated levels of circulating cytokines, which in turn promote bone resorption and suppress bone formation.8,9
Bisphosphonates belong to a class of compounds that exert a significant inhibitory effect on osteoclasts and are therefore potent antiresorptive agents.10 They reduce bone turnover, increase bone mineral density (BMD), and decrease fracture risk both at the lumbar spine and the hip. Due to their selectivity in action, they are usually not associated with systemic side effects or serious adverse events. The most frequently reported side effect attributed to the use of bisphosphonates is upper GI irritation. To date, nonrandomized studies 10–13 and one randomized, placebo-controlled trial14 have confirmed the benefit of daily therapy with oral bisphosphonates, including alendronate, in treating CF-related bone loss.
Despite the promise of therapy with oral bisphosphonates in CF patients, several concerns still exist.2 There is a need to determine the safety and tolerability of oral bisphosphonates in adults with CF who experience gastroesophageal reflux. In addition, there is an interest in determining the tolerability and adherence to weekly therapy (vs daily therapy) with oral bisphosphonate because of the demanding medical regimens of individuals with CF. To address these outstanding questions we conducted a multicenter, double-blind, placebo randomized controlled trial (RCT) of therapy with oral bisphosphonate alendronate (FOSAMAX; Merck; Whitehouse Station, NJ) administered once weekly over 12 months by adults with CF and low bone mass.
Materials and methods
Participants
Consenting and eligible adults with CF were recruited from six Canadian CF specialty clinics. Study recruitment began in December 2003 and was closed in August 2006. Participants ≥ 18 years of age with CF confirmed by positive sweat test result or DNA acid analysis and a BMD T score of ≤ − 1.0, as determined by dual-energy radiograph absorptiometry (DXA), were eligible for inclusion. Participants who had undergone organ transplantation; had endoscopy-proven esophagitis, gastritis, and ulceration; had metabolic bone disorders; had severe renal disease; had used systemic corticosteroids (dose, ≥ 7.5 mg/d) or other drugs known to influence bone metabolism in the previous 6 months; or had osteomalacia and other documented contraindications were excluded from the study. The protocol and study consent form received ethics review from McMaster University, and ethics approval was also obtained from each institution.
Study Protocol
Participants were randomized to receive placebo or oral alendronate, 70 mg once weekly for 12 months. The computer-generated randomization code, stratified according to institution, was prepared by an independent randomization center (McMaster In-Patient Pharmacy; Hamilton, ON, Canada), and block allocation was employed to ensure equitable distribution to each treatment group. The medication treatment arm was concealed, and all participants, central and local site coordinators, physicians, staff, and caregivers were blinded to treatment group allocation.
Participants were instructed to take their treatment medication while sitting upright and with water only on an empty stomach at least 30 min before first food or beverage of the day. In addition, all participants received 800 IU of vitamin D and 1,000 mg of calcium (500 mg supplementation, 500 mg from diet) daily. No (known) dose modifications were allowed during the course of the trial. Compliance was measured through pill counts at each visit and patient self-report during telephone contact. Participants who received at least 80% of the study drug were classified as being adherent to the protocol.14
In-clinic assessments were conducted at 6 and 12 months, and telephone follow-up was conducted by study staff at months 3 and 9. Intercurrent contact was also documented. Clinic assessments at baseline and 12 months included a physical examination, vital signs, biochemistry (serum and urine) tests, pulmonary function tests (including FEV1 and FVC), the Medical Outcomes Study 36-item short form, version 2 (SF-36v2),15 lateral radiographs of the thoracic and lumbar spine, and DXA to determine BMD. At baseline, calcium intake, diet history, and menstrual history were determined, and a serum pregnancy test was administered. At each contact, concomitant conditions and treatments were recorded, and patients were monitored for contraindications, pill counts were conducted, and instructions for taking the study medications were reviewed. In addition to spontaneous reporting, adverse events and drug reactions were elicited at each contact.
Safety analyses included all vertebral fractures, osteoporosis-related fractures, adverse reactions, and abnormal findings that had been detected through laboratory tests and physical examinations. Documentation for all adverse events were blinded and adjudicated by the external Data Safety Monitoring Committee. All adverse events were reported regardless of attribution to study medication. Stopping and study withdrawal rules were also monitored by this committee.
Bone Densitometry
BMD (lumbar spine and total hip) measurements were made in all participants at baseline and after 12 months using DXA. DXA scans were performed using either of two densitometers (Hologic Inc; Bedford, MA; or Lunar; Prodigy GE Healthcare, Waukesha, WI), and all DXA scan images were sent to a central DXA facility for analysis. A medical physicist (C.W.), who was blinded to the study treatment arm and study status, reviewed all DXA scans. Differences between DXA machines were identified by circulating a lumbar spine phantom to participating sites at the beginning and the end of the study, and by comparing the results of these measures.16 The adjustment of baseline BMD results according to the phantom spine measurements occurred for two cases. BMD measurements are reported in grams per square centimeter; T scores are also reported.
Fracture Determination Methods
A copy of the baseline and end-of-study lateral spine radiographs were sent to the central methods center, and were read independently by two radiologists (M.P. and J.O.) who were blinded to the study treatment arm. Radiographs were graded using the semiquantitative method of Genant et al.17 This method distinguishes fractured vertebrae (ie, > 20% compression; grades 1,2, and 3) from nonfractured vertebrae (ie, < 20% compression; grades 0 and 0.5). A deformity that was graded 1 and higher (excluding congenital or degenerative causes) was considered to be the minimum threshold for fracture in this study. Differences in scores between radiologists were resolved by consensus.
Statistical Analysis
Study data were entered and managed using database software (ACCESS; Microsoft; Redmond, WA). Analyses were performed with a statistical software package (SAS/STAT; SAS Institute Inc; Cary, NC). Clinical and laboratory variables were summarized as the mean (SD) and/or 95% confidence interval [CI]), or No. (%), as appropriate. All analyses were performed as intention-to-treat and included all available data.
The primary end point analysis was conducted using two-way analysis of variance, with the treatment and pooled center as fixed effects, and the difference in the percentage change in lumbar spine BMD at 12 months as the response variable. Secondary efficacy variables were analyzed using two-way analyses of variance, with treatment and pooled center as fixed effects and each secondary variable (ie, the change in total hip BMD and the change in SF-36v2 scores) as the response variable. Nonparametric statistics were used to compare discrete variables or outcomes between groups. Fractures and adverse event analyses were conducted by comparing the number of participants experiencing; an event between groups and the total number of events between groups.
Several potential confounding variables were examined in relation to the dependent variables. Confounding variables that were significant in univariate analysis (p < 0.1) were entered in multivariable regression analyses to examine the percentage change in lumbar spine and total hip BMDs over 12 months.
Results
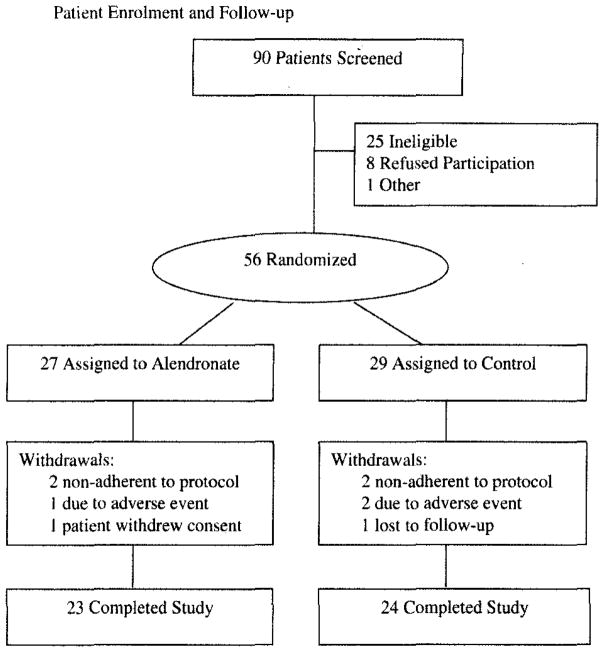
Of the 90 patients who were assessed for the study, 56 were enrolled. As displayed in Figure 1, 27 patients were randomized to the alendronate group and 29 were randomized to the control group, Overall, nine participants (16%) were withdrawn from the study; four in the alendronate group and five in the control group (Fig 1). An additional five participants completed the study protocol but received suboptimal dosing (< 80% adherence; alendronate group, three patients; control group, two patients). One of these participants (active group) missed > 50% of doses.
Figure 1.
Patient enrollment and follow-up.
Baseline characteristics were similar between groups (Table 1). Participants were mostly young adults with mild-to-moderate pulmonary disease as demonstrated by their baseline spirometric values and responses to the SF-36v2, Six participants were < 20 years of age at baseline. During the study, three participants in the treatment group used oral corticosteroids; the mean yearly cumulative dose was 49.32 mg, No one in the control group used oral corticosteroids during the study.
Table 1.
Baseline Characteristics*
| Characteristics | Alendronate Group (n = 27) | Control Group (n = 29) | Missing Cases (Alendronate Group/Control Group) |
|---|---|---|---|
| Gender† | |||
| Male | 17 (63) | 17 (59) | |
| Female | 10 (37) | 12 (41) | |
| Age, yr | 28.1 (7.7) | 30.9 (9.7) | 0/0 |
| Height, cm | 169.1 (10.3) | 165.3 (9.0) | 0/0 |
| Weight, kg | 57.8 (10.5) | 62.0 (12.3) | 0/0 |
| Body mass index, kg/m2 | 20.1 (2.0) | 22.6 (3.2) | 0/0 |
| FEV1 | |||
| L | 2.18 (0.9) | 1.98 (0.8) | 0/0 |
| % predicted | 58.1 (21.9) | 56.7 (20.7) | 0/0 |
| FVC | |||
| L | 3.50 (1.1) | 3.18 (1.1) | 0/0 |
| % predicted | 79.4 (18.6) | 79.9 (22.0) | 1/1 |
| Lumbar spine BMD, g/cm2 | 0.94 (0.12) | 0.95 (0.10) | 0/0 |
| Lumbar spine T score | −1.64 (0.71) | −1.68 (0.75) | 0/0 |
| Total hip BMD, g/cm2 | 0.88 (0.15) | 0.91 (0.11) | 0/0 |
| Total hip T–score | −1.01 (0.93) | −0.86 (0.65) | 0/0 |
| Calcium, mmol/L | 2.34 (0.11) | 2.29 (0.11) | 0/0 |
| Creatinine, μmol/L | 70.01 (11.33) | 69.91 (13.9) | 0/0 |
| Albumin, g/L | 40.9 (3.7) | 39.1 (3.6) | 0/0 |
| Alkaline phosphatase, U/L | 113.0 (72.7) | 107.4 (32.6) | 0/0 |
| 25-hydroxyvitamin D3 nmol/L | 60.9 (28.4) | 59.7 (28.0) | 3/3 |
| parathyroid hormone, pg/mL | 36.1 (17.0) | 45.9 (22.1) | 1/2 |
| SF-36v2† | |||
| PCS | 48.6 (6.4) | 46.2 (6.9) | 3/1 |
| MCS | 52.3 (9.0) | 47.0 (9.6) | 3/1 |
Values are given as the mean (SD), unless otherwise indicated. Standardized scores ranged from 0 to 100, with higher scores representing better function and quality of life.
Values are given as No. (%).
Bone Densitometry
At baseline, the mean (± SD) lumbar spine and total hip BMDs and T scores were similar between groups (Table 1). Primary outcome data were available for 23 participants (85.2%) in the alendronate group and 25 participants (86.2%) in the control group. The absolute percent changes in lumbar spine and total hip BMDs at follow-up were significantly greater in the alendronate group (5.20 ± 3.67% and 2.14 ± 3.32%, respectively) vs the control group (−0.08 ± 3.93% and −1.3 ± 2.70%, respectively; p < 0.001). In multivariable analyses (controlling for trial site, baseline serum calcium, baseline FEV1, number of prevalent vertebral fractures, age, and baseline BMD), after 12 months the alendronate group demonstrated a 4.04% greater increase (95% CI, 1.71 to 6.37%; P = 0.001) in lumbar spine BMD compared to the control group and a 3.03% greater increase (95% CI, 1.24 to 4.81; P = 0.002) in total hip BMD compared to the control group. At 12 months, 21 participants (91.3%) and 16 participants (69.6%), respectively, in the alendronate group demonstrated a > 0 increase in lumbar spine and total hip BMDs vs 13 participants (52.0%) and 6 participants (24.0%) in the control group (p < 0.01).
Vertebral Fractures
At baseline, three participants in the alendronate group and six participants in the control group demonstrated at least one grade 1 (n = 14) or grade 2 (n = 1) vertebral fracture. The 12-month vertebral fracture data were available for 23 participants (85.2%) in the alendronate group and 24 participants (82.8%) in the control group. Of these participants, no new grade 1 or grade 2 fractures were experienced in the alendronate group, and two participants in the control group experienced a new grade 1 fracture (difference not significant). All fractures occurred in the thoracic spine.
Quality of Life
The SF-36v2 physical component score (PCS) and mental component score (MCS) were similar at baseline between groups (Table 1). At follow-up, the mean changes in PCS and MCS were −1.18 ± 4.93 and −2.67 ± 7.55, respectively, in the alendronate group vs −3.69 ± 8.33 and 3.26 ± 12.27, respectively, in the control group. While these changes were not significant between groups, the mean baseline and follow-up PCSs for the entire group and for each treatment group fell below the mean age-standardized and sex-standardized scores for Canadians.18
Safety Analysis
Table 2 presents the proportion of participants in the alendronate group and the control group who experienced adverse events, including serious events and drug-related events. Overall, seven participants (25.9%) in the alendronate group and three participants (10.3%) in the control group experienced a serious adverse event (difference not significant). Serious adverse events in the alendronate group were classified as exacerbation of CF (n = 3), bronchial superinfection (n = 1), hypoglycemic seizure (n = 1), GI (n = 1), and intestinal obstruction (n = 1). Three participants who experienced serious adverse events in the control group were classified as having an exacerbation of CF, with two of these participants reporting additional GI complaints. Table 3 provides a description of all GI-related adverse events that occurred over the course of the study. Ten GI-related events occurred in the alendronate group, and seven GI events occurred in the control group. The most common GI-related adverse events were nausea and/or vomiting.
Table 2.
Number of Participants With One or More Adverse Event*
| Variables | Alendronate Group (n = 27)
|
Control Group (n = 29)
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Any AE | 15 | 56 | 19 | 66 |
| Serious AE | 7† | 26 | 3‡ | 10 |
| Drug-related AE | 8 | 30 | 8 | 28 |
AE = adverse event.
In the alendronate group, three patients were classified as having had an exacerbation of CF, one patient was identified as having a bronchial superinfection, one patient was identified as having a hypoglycemic seizure, one patient was identified as having a GI obstruction, and one patient was identified as having an intestinal obstruction.
In the control group, all three participants were classified as having an exacerbation of CF, with two of them having additional GI complaints.
Table 3.
Total GI-Related AEs*
| AEs | Alendronate Group (n = 27) | Control Group (n = 29) |
|---|---|---|
| Nausea and/or vomiting | 3 | 4 |
| Reflux | 1 | 0 |
| Difficulty swallowing | 1 | 0 |
| Esophagitis | 1 | 0 |
| Constipation | 1 | 1 |
| GI upset | 1 | 0 |
| Intestinal obstruction | 1 | 1 |
| Stomach pain/burn | 1 | 1 |
More than one event could have occurred per participant. See Table 2 for abbreviation not used in the text.
Discussion
The bisphosphonate alendronate is an oral anti-resorptive agent that is commonly used to treat osteoporosis. To date, its greatest success has been for use in postmenopausal women, 10,19–22 men > 65 years of age, 23–25 and patients with corticosteroid-induced osteoporosis.26,27 Improvements in BMD range from 2 to 6%, which are considered clinically important and are statistically significant when compared to BMD changes in the control arm of randomized trials. Other smaller, nonrandomized studies11–13 and one RCT14 have also confirmed the benefit of therapy with bisphosphonates in treating CF-related bone loss. RCTs of IV bisphosphonates also have demonstrated28,29 significant BMD improvements in CF participants; however, a common treatment side effect is infusion-related bone pain. As a result, oral bisphosphonates are considered to be first-line therapy for those persons who are identified to be at risk in this population.2,3
In our study, we examined therapy with alendronate, 70 mg once weekly, and found clinically significant improvements in BMD over 12 months. Compared with the control group, the alendronate group had 4.04% greater BMD gain at the lumbar spine and 3.03% greater BMD gain at the total hip even after controlling for other variables. We did not have the power to properly examine new vertebral fractures. Two patients in the control group and none in the treatment group experienced a new vertebral fracture after the baseline measurement.
Our study has results that were similar to those of an RCT by Aris and colleagues.14 In that study, daily alendronate was examined in a similar cohort of CF participants. At 12 months, the alendronate group had increased BMD by 4.9% in the spine and 2.8% in the femur compared to the control group, which lost BMD at both sites. These findings are similar to what we observed. The treatment group demonstrated a 5.2% increase over baseline in lumbar spine BMD and a 2.1% increase in BMD of the total hip. The proportion of participants who adhered to alendronate treatment was similar in both studies.
Overall, the safety profile of alendronate in this study was favorable; the number of patients who experienced a drug-related adverse event was equal between the alendronate and control groups (eight patients in each group), and the number of occurrences of GI-related adverse events was similar (alendronate group, 10 occurrences; control group, 7 occurrences). However, it should be noted that a serious adverse event occurred in one patient receiving alendronate that was GI in nature (eg, nausea, vomiting, and GI discomfort) and may have been attributable to the study drug. In general, the potential risk of adverse effects may be higher in this population and needs to be monitored. Furthermore, prescribing bisphosphonates to women with child-bearing potential needs to be considered carefully, since therapy with bisphosphonates is not recommended in pregnant women.
One of the limitations of our study is that it was difficult to recruit patients, and we were challenged by the relatively small sample size. We did not see a statistically significant difference in the rate of new vertebral fractures in the treatment group vs the control group. When the results of this study and those of Aris et al14 are considered together, the findings clearly indicate that daily or weekly alendronate treatment increases BMD in young adults with CF.
As in patients with primary osteoporosis, the greatest number of fractures occur in those with osteopenia and not with osteoporosis, while the relative fracture risk is greatest in those with osteoporosis.30 This suggests that factors other than BMD need to be taken into consideration when determining fracture risk. In CF patients, other factors such as vitamin D deficiency and calcium malabsorption, poorer health status, and the disease itself may be additional risk factors for the occurrence of fractures. These factors need to be taken into consideration when contemplating treatment. The decline in BMD over 1 year suggests that the control group had accelerated bone loss. Given this decline, we would recommend that a BMD test be performed annually or every second year, and that it include an assessment of gonadal status, with intervention considered if accelerated bone loss persists despite conservative therapy with exercise, calcium, and vitamin D. Anyone who is likely to require an organ transplant in the next few years should also be considered for treatment, given that these patients are at high risk for fracture in the year following the transplant.31
Conclusion
In this multicenter, double-blind trial of young adults with CF and low bone mass, treatment with alendronate (70 mg once weekly) was well tolerated and demonstrated a clinically Significant increase in BMD over 12 months compared with placebo. In the control arm, daily administration of vitamin D (800 IU) and calcium (1,000 mg) demonstrated no improvement of BMD over 12 months. The number of side effects was similar in both groups, suggesting that alendronate is a safe and effective treatment for CF-related bone disease.
Acknowledgments
Study funding was provided by Merck Frosst Canada.
The authors thank the participants for their commitment to the study; Nicole Ferko and Christine Brenckman for assisting in protocol development; the dedicated team at the Central Site, including Anjali Pathak and Ruth McCallum; and the site coordinators, including Rosamund Hennessey, Suzanne Hansen, Nadia Beaudoin, Patrice Kean, Jeanette Leong, Dr. Reinhard Kloiber, France Paquet, and Chantale Savard, for the collection of study data in a rigorous and timely manner. The authors would like to acknowledge Dr. Robert Josse and Dr. Angela Cheung for their participation on the external Data Safety Monitoring Committee. We thank Dr. Jacques Brown for providing the analysis of biomarkers. We also thank Annette Wilkins for her assistance with study closeout and manuscript preparation.
Abbreviations
- BMD
bone mineral density
- CF
cystic fibrosis
- CI
confidence interval
- DXA
dual-energy x-ray absorptiometry
- MCS
mental component score
- PCS
physical component score
- RCT
randomized controlled trial
- SF-36v2
Medical Outcomes Study 36-item short form, version 2
Footnotes
Patients were recruited for the study from speciality clinics at McMaster University, Hamilton, ON, Canada; Centre Hospitalier de l’Université de Montréal, Montréal, QC, Canada; University of Calgary, Calgary, AB, Canada; London Health Science Centre, London, ON, Canada; Montreal Chest Institute, Montréal, QC, Canada; and Le Centre Hospitalier Universitaire de Québec, Québec City, QC, Canada. Centralized analyses of bone marker samples, radiograph readings, bone mineral density readings and statistical analyses were performed at the following: McMaster University; and Laval University Medical Center (CHUL), Québec City, QC, Canada.
Dr. Papaioannou has had consulting and advisory roles for Amgen, Eli Lilly, Merck Frosst, Novartis, Proctor & Gamble, sanofi-aventis, and Servier; she has participated in clinical trials sponsored by Amgen, Eli Lilly, Merck, Novartis, Proctor & Gamble, and sanofi-aventis. Dr. Adachi has had consulting roles for Amgen, Astra Zeneca, Eli Lilly, GlaxoSmithKline, Merck Frosst, Novartis, Proctor & Gamble, Roche, sanofi-aventis, and Servier; he has participated in clinical trials sponsored by Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Proctor & Gamble, sanofi-aventis, Servier, and Wyeth-Ayerst. Ms. Kennedy, Dr. Freitag, Mr. Ioannidis, Dr. O’Neill, Dr. Webber, Dr. Pui, Dr. Berthiaume, Dr. Rabin, Dr. Paterson, Dr. Jeanneret, Dr. Matouk, Dr. Villeneuve, and Ms. Nixon have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Strausbaugh SD, Davis PB. Cystic fibrosis: a review of epidemiology and pathobiology. Clin Chest Med. 2007;28:279–288. doi: 10.1016/j.ccm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Aris RM, Merkel PA, Bachrach LK, et al. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90:1888–1896. doi: 10.1210/jc.2004-1629. [DOI] [PubMed] [Google Scholar]
- 3.Boyle MP. Update on maintaining bone health in cystic fibrosis. Curr Opin Pulm Med. 2006;12:453–458. doi: 10.1097/01.mcp.0000245708.59138.a4. [DOI] [PubMed] [Google Scholar]
- 4.Hecker TM, Aris RM. Management of osteoporosis in adults with cystic fibrosis. Drugs. 2004;64:133–147. doi: 10.2165/00003495-200464020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Papaioannou A, Parkinson W, Ferko N, et al. Prevalence of vertebral fractures among patients with chronic obstructive pulmonary disease in Canada. Osteoporos Int. 2003;14:913–917. doi: 10.1007/s00198-003-1449-5. [DOI] [PubMed] [Google Scholar]
- 6.Orens JB, Estenne M, Arcasoy S, et al. International guide-lines for the selection of lung transplant candidates: 2006 update; a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Yankaskas JR, Mallory GB., Jr Lung transplantation in cystic fibrosis: consensus conference statement. Chest. 1998;113:217–226. doi: 10.1378/chest.113.1.217. [DOI] [PubMed] [Google Scholar]
- 8.Haworth CS, Selby PL, Webb AK, et al. Inflammatory related changes in bone mineral content in adults with cystic fibrosis. Thorax. 2004;59:613–617. doi: 10.1136/thx.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shead EF, Haworth CS, Gunn E, et al. Osteoclastogenesis during infective exacerbations in patients with cystic fibrosis. Am J Respir Crit Care Med. 2006;174:306–311. doi: 10.1164/rccm.200512-1943OC. [DOI] [PubMed] [Google Scholar]
- 10.Lambrinoudaki I, Christodoulakos G, Botsis D. Bisphosphonates. Ann N Y Acad Sci. 2006;1092:397–402. doi: 10.1196/annals.1365.036. [DOI] [PubMed] [Google Scholar]
- 11.Cawood TJ, McKenna MJ, Gallagher CG, et al. Oral bisphosphonates improve bone mineral density in adults with cystic fibrosis. Ir Med J. 2005;98:270–273. [PubMed] [Google Scholar]
- 12.Conway SP, Oldroyd B, Morton A, et al. Effect of oral bisphosphonates on bone mineral density and body composition in adult patients with cystic fibrosis: a pilot study. Thorax. 2004;59:699–703. doi: 10.1136/thx.2002.002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringuier B, Leboucher B, Leblanc M, et al. Effect of oral biphosphonates in patients with cystic fibrosis and low bone mineral density. Arch Pediatr. 2004;11:1445–1449. doi: 10.1016/j.arcped.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Aris RM, Lester GE, Caminiti M, et al. Efficacy of alendronate in adults with cystic fibrosis With low bone density. Am J Respir Crit Care Med. 2004;169:77–82. doi: 10.1164/rccm.200307-1049OC. [DOI] [PubMed] [Google Scholar]
- 15.Ware J, Kosinski M, Dewey J. How to score version two of the SF-36 health survey. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 16.Genant HK. Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res. 1995;10:997–998. doi: 10.1002/jbmr.5650100624. [DOI] [PubMed] [Google Scholar]
- 17.Genant HK, Li J, Wu CY, et al. Vertebral fractures in osteoporosis: a new method for clinical assessment. J Clin Densitom. 2000;3:281–290. doi: 10.1385/jcd:3:3:281. [DOI] [PubMed] [Google Scholar]
- 18.Hopman WM, Towheed T, Anastassiades T, et al. Canadian normative data for the SF-36 health survey: Canadian Multicentre Osteoporosis Study Research Group. Can Med Assoc J. 2000;163:265–271. [PMC free article] [PubMed] [Google Scholar]
- 19.Qin L, Choy W, Au S, et al. Alendronate increases BMD at appendicular and axial skeletons in patients with established osteoporosis. J Orthop Surg. 2007;2:9. doi: 10.1186/1749-799X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen CJ, Hochberg MC, Bonnick SL, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly, risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151. doi: 10.1359/JBMR.040920. [DOI] [PubMed] [Google Scholar]
- 21.Bone HG, Hosking D, Devogelaer JP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 22.Bonnick S, Broy S, Kaiser F, et al. Treatment with alendronate plus calcium, alendronate alone, or calcium alone for postmenopausal low bone mineral density. Curr Med Res Opin. 2007;23:1341–1349. doi: 10.1185/030079907X188035. [DOI] [PubMed] [Google Scholar]
- 23.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto J, Takeda T, Sato Y, et al. Comparison of the effect of alendronate on lumbar bone mineral density and bone turnover in men and postmenopausal women with osteoporosis. Clin Rheumatol. 2007;26:161–167. doi: 10.1007/s10067-006-0252-z. [DOI] [PubMed] [Google Scholar]
- 25.Sawka AM, Papaioannou A, Adachi JD, et al. Does alendronate reduce the risk of fracture in men? A meta-analysis incorporating prior knowledge of anti-fracture efficacy in women. BMC Musculoskelet Disord. 2005;6:39. doi: 10.1186/1471-2474-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lems WF, Lodder MC, Lips P, et al. Positive effect of alendronate on bone mineral density and markers of bone turnover in patients with rheumatoid arthritis on chronic treatment with low-dose prednisone: a randomized, double-blind, placebo-controlled trial. Osteoporos Int. 2006;17:716–723. doi: 10.1007/s00198-005-0037-2. [DOI] [PubMed] [Google Scholar]
- 27.de Nijs RN, Jacobs JW, Lems WF, et al. Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med. 2006;355:675–684. doi: 10.1056/NEJMoa053569. [DOI] [PubMed] [Google Scholar]
- 28.Haworth CS, Selby PL, Webb AK, et al. Severe bone pain after intravenous pamidronate in adult patients with cystic fibrosis. Lancet. 1998;352:1753–1754. doi: 10.1016/S0140-6736(05)79826-3. [DOI] [PubMed] [Google Scholar]
- 29.Haworth CS, Selby PL, Adams JE, et al. Effect of intravenous pamidronate on bone mineral density in adults with cystic fibrosis. Thorax. 2001;56:314–316. doi: 10.1136/thorax.56.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–1112. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- 31.Shane E, Papadopoulos A, Staron RB, et al. Bone loss and fracture after lung transplantation. Transplantation. 1999;68:220–227. doi: 10.1097/00007890-199907270-00010. [DOI] [PubMed] [Google Scholar]