Abstract
A homologous series of pore-forming amphiphiles (PFAs), derived from cholic acid, lysine and spermine, have been used as “thermal-gates” for releasing sucrose from liposomes made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-dipalmitoyl-sn-glycero-e-[phospho-rac(1-glycerol)] (sodium salt) [DPPG]. Binding measurements have established that these PFAs are fully bound to these liposomes in their gel state, and that their transfer to fluid phase membranes is negligible. Release experiments have shown that thermal-gating is sensitive to both the size and the concentration of the PFA that is used. Increases in the extent of release of sucrose with increasing temperature that have been found in the gel/fluid coexistence region indicate the existence of heterogeneity among the liposomes.
INTRODUCTION
Finding ways to control the release of encapsulated agents from the aqueous interior of liposomes continues to attract broad interest from an applications standpoint (1-22). Although most of this interest has centered around drug delivery, a variety of other applications can be considered as well; e.g., the release of catalysts, reagents, cosmetics, flavors, dyes, fragrances, contrast agents, etc.
One well-established approach for controlling the release of liposome-encapsulated agents takes advantage of differences in membrane permeability between the gel (i.e., solid-like) and the liquid-crystalline (i.e., fluid-like) state. In particular, whereas gel-phase bilayers are characterized by tight lipid packing and low permeability, fluid phase analogs are loosely packed and have relatively high permeabilities (23-26). Thus, by increasing the temperature of liposomes beyond their gel to liquid-crystalline phase transition temperature (Tm), one can observe increased permeation rates (24-26). In some instances, it has been noted that maximum rates occur at or near the lipid's Tm, where half of the membrane has melted and gel and fluid phases coexist (27). Although the reason for this maximum has not been firmly established, it has been suggested that a mismatch in lipid packing between the two phases produces defects through which permeants can pass (27).
Previously, we introduced the concept of “thermally-gated liposomes”, whereby a pore-forming agent (PFA) derived from facially amphiphilic cholic acid is used to enhance thermally-induced liposomal release (28). Our working hypothesis is that these PFAs assemble into pores either in the fluid phase or at gel/fluid interfaces (Figure 1).
Figure 1.
Stylized illustration showing two PFAs forming a pore in a melted bilayer.
In the work reported herein, we sought to gain insight into the interactions between PFAs and gel phase liposomes, and to better characterize the thermally-gated release process. Of particular interest was to determine whether PFAs are fully bound to gel phase liposomes, whether they are transferrable to fluid phase analogs, and whether thermally-gated release is dependent on the size of the PFA that is used. We also sought to distinguish between thermally-gated release occurring in the fluid phase versus gel/fluid interfaces.
In earlier studies, we showed that a non-facially amphiphilic, ion conductor is “squeezed out” of liposomal membranes and released into the aqueous phase when the bilayer is converted from the fluid phase to the gel state (29). If PFAs derived from cholic acid were to behave similarly, this would limit their usefulness in drug delivery, since they would be expected to undergo competitive binding to cell membranes. For this same reason, transfer of a PFA from gel phase liposomes to fluid phase membranes via direct membrane-membrane contact would be a significant limitation. Thus, the inherent stability of thermally-gated liposomes in the presence of fluid membranes has practical implications. Equally important from a drug delivery standpoint is the rate at which encapsulated agents are released from liposomes. Since release rates approaching “burst kinetics” are most suitable for drug targeting via localized hyperthermia, knowing whether release is faster in the fluid phase or in the gel/fluid coexistence region has practical relevance. In addition, knowing whether the size of a PFA significantly influences release rates is an important consideration. Although we have shown that the activity of a PFA for Na+ transport increases, exponentially, with increasing size, whether the size of a PFA influences the rate of release of molecules from liposomes under thermally-gated conditions remains to be determined (28).
RESULTS
Experimental Design
To examine these issues, we employed liposomal carriers (ca. 200 nm diameter, extrusion) made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and a small amount (5 mol%) of the negatively charged analog, 1,2-dipalmitoyl-sn-glycero-e-[phospho-rac(1-glycerol)] (sodium salt) [DPPG]. The latter was included in these dispersions to stabilize them by preventing aggregation. Extrusion procedures used in forming thermally-gated liposomes (200 nm Nuclepore membranes) are described in the Supporting Information. Because DPPC and DPPG have a Tm of 41°C, which is slightly above physiological 37°C, these liposomes could be considered for drug delivery.
Three pore-forming agents that were chosen for this study were derived from cholic acid, lysine and spermine (Figure 2). The largest one (i.e., 1) contains 12 choloyl groups coupled to a spermine backbone that has been extended at each end by one lysine group (30). An intermediate-sized analog (i.e., 2) has a total of 8 choloyl groups bound to this same framework (30). Finally, a relatively small analog (i.e., 3) has only 4 choloyl groups bound to spermine (31). In this work, sucrose was chosen as a model permeant because it is uncharged and highly water soluble.
Figure 2.
Structures of pore-forming amphiphiles and permeant (sucrose) used in this study.
Thermotropic Properties
Before carrying out thermally-gated release experiments, we first examined the melting properties of our liposomes in the absence and in the presence of 1, 2 and 3 by high-sensitivity differential scanning calorimetry (hs-DSC). For these measurements, and for thermally-gated release experiments, a low concentration of PFA was used; that is, 1.2 mol% choloyl groups, corresponding to 0.1, 0.15 and 0.3 mol % of 1, 2 and 3, respectively.
In the absence of PFAs, a broad pre-transition was observed at ca. 35°C. In addition, the main gel to liquid-crystalline phase transition appeared as a doublet, centering at ca. 41°C (Figure 3). It should be noted that similar splitting has been reported for other phosphocholine-based liposomes when their diameters are less than ca. 200 nm (32). In the presence of each of these PFAs, the pre-transition was essentially eliminated and the main phase transition was converted into a sharp single endotherm.
Figure 3.
High-sensitivity differential scanning calorimetry data for extruded liposomes made from DPPC/DPPG (95/5, mol/mol) with (A) no PFA added, (B) 0.1 mol% of 1, (C) 0.15 mol% of 2, and (D) 0.3 mol% of 3.
Binding of PFAs to Gel Phase Liposomes
To judge the extent of binding of each PFA by gel phase liposomes, we determined relative concentrations in dispersions before and after gel filtration by quantitative thin layer chromatography. For these analyses, the DPPG that was present in the liposomes was used as an internal standard. Since the ratio of PFA/DPPG was essentially the same before and after gel filtration in all cases, these results indicate that these PFAs are fully bound (± ca. 5%) to these gel phase liposomes at 23°C (Table 1).
Table 1.
Quantification of liposome-bound PFAs before and after gel filtration.a
| PFA | Integrated density of TLC spots | |||||
|---|---|---|---|---|---|---|
| Before gel-filtration | After gel-filtration | |||||
| PFA | DPPG | Ratio of PFA/DPPG |
PFA | DPPG | Ratio of PFA/DPPG |
|
| 1 | 107423 | 401666 | 0.27 | 79194 | 315235 | 0.25 |
| 2 | 197958 | 919363 | 0.22 | 203264 | 885709 | 0.23 |
| 3 | 125934 | 755700 | 0.17 | 75604 | 470334 | 0.16 |
Integrated densities measured for PFAs and DPPG are in arbitrary units.
Attempted Transfer of a PFA from Gel to Fluid Phase Liposomes
In an attempt to transfer a PFA from gel phase liposomes to fluid analogs, we mixed an extruded dispersion made from DPPC/DPPG (95/5, mol/mol) containing 0.3 mol% of 3 with multilamellar vesicles (MLVs) made from 1-palmitoyl, 2-oleoyl-sn-glycero-3-phosphocholine (POPC, Tm=−2.6°C) (33, 34). After an incubation period of 16 h at 37°C with 10 equivalents of POPC, the resulting dispersion was cooled to room temperature and centrifuged to pellet the MLVs. Under these conditions, the extruded liposomes remain dispersed. Analysis of the ratio of 3/DPPG in the supernatant by quantitative TLC indicated no loss of 3 from the gel phase liposomes.
Thermally-Gated Release of Sucrose
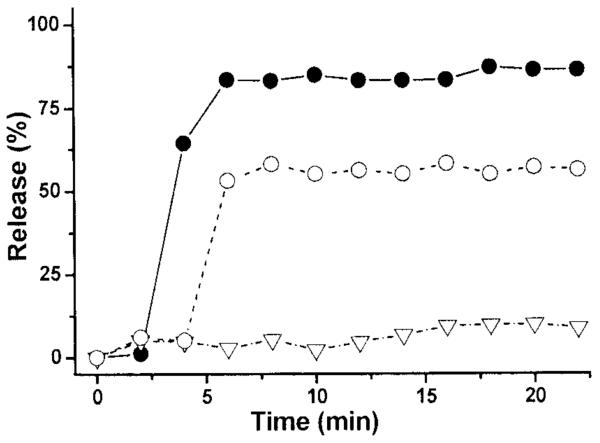
To test for thermal-gating, sucrose was encapsulated in liposomes containing 1, 2 or 3. After gel filtration to remove external sucrose, the liposomes were then immediately heated to either 37°C or 43°C. Aliquots were withdrawn as a function of time and rapidly gel filtered a second time to remove the sucrose that had been released. The amount of sucrose that was retained by the liposomes was quantified by spectrophotometry after digestion (see Supporting Information). In Figure 4 are shown the release profiles. In the absence of a PFA, the release of sucrose was negligible at both of these temperatures. When 1 was included in the liposomes, however, the release was rapid and complete at 43°C. Somewhat unexpectedly, a similar release profile was observed at 37°C. When 2 was used as the PFA, the extent of release was nearly complete at both temperatures, but was apparently slower at 37°C. In contrast to 1 and 2, the use of 3 as the PFA resulted in thermal-gating; that is, there was negligible release at 37°C and rapid and substantial release at 43°C.
Figure 4.
Plot of percent of sucrose released as a function of time from liposomes made from DPPC/DPPG (95/5, mol/mol) with (A) no PFA added, (B) 0.1 mol% of 1, (C) 0.15 mol% of 2, and (D) 0.3 mol% of 3 at (▽) 37°C and (●) at 43°C.
The difference in release rates for 2 at 37°C versus 43°C suggested that an even greater difference might be observed using a lower concentration of the PFA. Consistent with this thinking, when only 0.015 mol% of 2 was used, the extent of release at 37°C was negligible; however, at 43°C, a rapid and significant release was observed (Figures 5). Qualitatively, similar behavior was observed when 0.010 mol% of 1 was used.
Figure 5.
Release of sucrose as a function of time from liposomes made from DPPC/DPPG (95/5, mol/mol) containing (A) 0.010 mol% of 1 and (B) 0.015 mol% of 2 at (▽)37°C and (●) 43°C.
Dynamic light scattering measurements that were made for liposomes containing 0.10 mol% of 1, 0.15 mol% of 2, and 0.30 mol % of 3 showed diameters before (and after) thermally gated release that were 164 ± 59 nm (164 ± 62 nm), 150 ± 57 nm (150 ± 60 nm), and 148 ± 59 nm (148 ± 56 nm), respectively.
In an effort to determine whether thermally-gated release is favored in the fluid phase or the gel/fluid coexistence region, a similar set of experiments was carried out using 3 at 41°C and at 43°C; that is, at the Tm of the bilayer, and at a temperature in which the bilayer is fully melted. Thus, if release were favored in the gel/fluid coexistence region, the efflux rate should be greater at 41°C than at 43°C; if release were favored in the fluid phase, then a greater efflux rate should be observed at 43°C. Although the release profiles at 41°C showed a slight time lag for the onset of release, the similarity in the slopes at both temperatures does not allow a clear distinction to be made between preferred release in fluid phase versus the gel/fluid coexistence region (Figure 6). One interesting difference between these release profiles is the lower extent of release at the lower temperature. To confirm this behavior, we prepared a similar dispersion and divided it into 10 portions. Each portion was heated for 6 min at temperatures ranging between 36.5°C and 43.5°C, and the extent of release was determined. As shown in Figure 7, a maximum release was found when the membrane was fully melted.
Figure 6.
Release of sucrose as a function of time from liposomes made from DPPC/DPPG (95/5, mol/mol) containing 0.3 mol% of 3 at (▽) 37°C, (○) 41°C, and (●) 43°C.
Figure 7.
Release of sucrose from liposomes made from DPPC/DPPG (95/5, mol/mol) containing 0.3 mol% of 3 as a function of temperature using a 6 min incubation period.
Further evidence for greater release occurring when the liposomes were fully melted was obtained by dividing a dispersion containing 0.3 mol% of 3 into two portions. One portion was rapidly heated to 41.5°C and held at that temperature for 6 min and cooled to room temperature. This heating/cooling cycle was then repeated five additional times and the extent of sugar release was analyzed after each cycle. The second portion of liposomes was heated only once to 43°C and held for 6 min before cooling to room temperature. As shown in Figure 8, only a modest increase in the amount of sucrose that was released was observed with additional heating/cooling cycles at 41.5°C. In contrast, the extent of release at 43°C was significantly greater.
Figure 8.
Release of sucrose from liposomes made from DPPC/DPPG (95/5, mol/mol) containing 0.3 mol% of 3 as a function of the number of heating/cooling cycles at 41.5 °C (○), and from one heating period at 43 °C (●); in all cases 6 min incubation periods were used.
Discussion
Analysis of the gel phase liposomes used in this study by hs-DSC, in the absence of PFAs, showed a main phase transition that appeared as a doublet, which is similar to observations that have been made by other researchers for highly curved phosphocholine-based liposomes (32). Although the basis for this splitting remains to be established, one possibility is that the inner-concave and outer-convex leaflets have distinct melting characteristics due to differences in packing. Regardless of its origin, the addition of very small amounts of 1, 2 and 3 was sufficient to promote greater cooperativity in the melting process, and the conversion to a sharp single endotherm. We suspect that this effect may be due to a “filling in” of gaps within each of the leaflets by the PFA.
Binding measurements that were made for 1, 2 and 3 have shown that these PFAs are fully bound to gel phase liposomes made from DPPC/DPPG (95/5, mol/mol), and that the transfer of 3 to a fluid phase analog is negligible. Thus, thermally-gated liposomes would appear to be reasonably stable.
The dependency of thermally-gated release on the size of the PFA that is used was found to be significant. The fact that 1 and 2 release almost all of the entrapped sucrose at 37°C (when 0.10 and 0.15 mol% are used, respectively), but release very little when their concentration is reduced by one order of magnitude, suggests that aggregates may be responsible for release from gel phase liposomes. A previous study of 1 and 2 as agents for promoting the transport of Na+ across fluid bilayers has shown that two PFAs combine to form a pore (28, 30). If the release of sucrose is favored in fluid regions of the membrane under thermal gating conditions, then a similar pore structure may be involved.
The fact that the maximum amount of sucrose, which is released under thermal gating conditions, is dependent on temperature, and that repetitive heating/cooling cycles at a fixed temperature in the gel/fluid coexistence region barely increase the extent of this release, indicates that there is heterogeneity among the liposomes; that is, some of liposomes release their contents at a given temperature, while others do not. The origin of this heterogeneity is not presently clear. One model that can be considered is based on the following assumptions: (i) the PFAs are nonuniformly distributed among the liposomes, (ii) the effective concentration of a PFA within a liposome is determined by its total concentration in the membrane, and by the fraction of the membrane that has been converted into the fluid phase, (iii) those PFAs that reside in gel regions of the membrane do not form pores at sufficiently low concentrations, and (iv) small aggregates of PFAs are formed in fluid regions, and it is these pores that are responsible for thermally-gated release. Thus, at a given temperature in the gel/fluid coexistence region, some of the liposomes have a sufficient concentration of PFA in fluid regions to form pores, which release sucrose. As the temperature is increased and a greater percentage of the membranes are melted, the concentration of PFAs in fluid regions is then increased, and the release of sucrose from those liposomes containing lesser amounts of PFA is possible.
The finding that less than 100% of sucrose is released even at 43°C (under thermal gating conditions) is consistent with a non-uniform distribution of PFAs among the liposomes. The fact that at 43°C, 100% release was found using 0.1 mol% of 1, slightly less than 100% release was found using 0.15 mol% of 2, and significantly less than 100% release was observed using 3 suggests that the larger PFAs are more efficient in releasing sucrose, if one assumes that their distribution among the liposomes is similar. From an operational standpoint, the fact that of 0.015 mol% of 2 shows thermal gating behavior that is very similar to that found with 0.3 mol% of 3, indicates that a main virtue of the use of larger PFAs is that much lower concentrations can be employed.
Studies that are currently in progress are aimed at determining (i) the size of the pores that are created under thermal-gating conditions by examining the release of entrapped sugars of varying size, and (ii) whether aggregates of such amphiphiles produce pores when these membranes have been converted to the fluid phase by means of kinetic measurements (30).
Conclusions
Experimental evidence has been obtained, showing that PFAs 1, 2 and 3 are fully bound to gel-phase liposomes made from DPPC/DPPG (95/5, mol/mol), that transfer of a PFA from gel phase liposomes to fluid phase analogs is negligible, and that thermally-gated release is sensitive to both the size as well as the concentration of the PFA that is used. Relatively high concentrations of PFAs favor the release of entrapped sucrose from gel phase liposomes, suggesting that aggregates may be responsible. An increase in the extent of sucrose release with increasing temperatures within the gel/fluid coexistence region has also revealed heterogeneity among the liposomes. The question of whether thermally-gated release is favored in the fluid phase or the gel/fluid coexistence region has not been answered, unambiguously, although the present findings suggest that the fluid phase may be more likely.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the National Science Foundation (CHE-0345248) and to the National Institutes of Health (PHS Grant GM51814) for support of this research.
Footnotes
SUPPORTING INFORMATION. Experimental procedures. This information is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 2.Dreher MR, Chilkoti A. Toward a systems engineering approach to cancer drug delivery. J. Natl. Cancer Inst. 2007;99:983–985. doi: 10.1093/jnci/djm042. [DOI] [PubMed] [Google Scholar]
- 3.Guo X, Szoka FC. Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc. Chem. Res. 2003;36:335–341. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 4.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjugate Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andresen TL, Jensen SS, Kaasgaard T, Jorgensen K. Triggered activation and release of liposomal prodrugs and drugs in cancer tissue by secretory phospholipase A2. Curr. Drug Delivery. 2005;2:353–362. doi: 10.2174/156720105774370203. [DOI] [PubMed] [Google Scholar]
- 6.Fattal E, Couvreur P, Dubernet C. “Smart” delivery of antisense oligonucleotides by anionic pH-sensitive liposomes. Adv. Drug Delivery Rev. 2004;56:931–946. doi: 10.1016/j.addr.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Ong W, Yang Y, Cruciano AC, McCarley RL. Redox-triggered contents release from liposomes. J. Am. Chem. Soc. 2008;130:14739–14744. doi: 10.1021/ja8050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Cytosolic drug delivery using pH- and light-sensitive liposomes. Adv. Drug Delivery Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 9.Davidsen J, Jorgensen K, Andresen TL, Mouritsen OG. Secreted phospholipase A2 as a new enzymatic trigger mechanism for localized liposomal drug release and adsorption in diseased tissue. Biochim. Biophys. Acta. 2003;1609:95–101. doi: 10.1016/s0005-2736(02)00659-4. [DOI] [PubMed] [Google Scholar]
- 10.Meers P. Enzyme-activated targeting of liposomes. Adv. Drug Delivery Rev. 2001;53:265–272. doi: 10.1016/s0169-409x(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 11.Huang Z, Szoka FC. In: In Liposome Technology. 3rd ed. Gregoriadis G, editor. Vol. 3. CRC Press; Boca Raton, FL: 2006. pp. 165–196. [Google Scholar]
- 12.Sarkar N, Banerjee J, Hanson AJ, Elegbede AI, Rosendahl T, Krueger AB, Banerjee AL, Tobwala S, Wang RY, Lu XN, Mallik S, Srivastava DK. Matrix metalloproteinase-assisted triggered release of liposomal contents. Bioconjugate Chem. 2008;19:57–64. doi: 10.1021/bc070081p. [DOI] [PubMed] [Google Scholar]
- 13.Chandra B, Mallik S, Srivastava DK. Design of photo-cleavable lipids and their applications in liposomal “uncorking”. Chem. Commun. 2005:3021–3023. doi: 10.1039/b503423j. [DOI] [PubMed] [Google Scholar]
- 14.Eastoe J, Vesperinas A, Donnewirth AC, Wyatt P, Grillo I, Heenan RK, Davis S. Photodestructible vesicles. Langmuir. 2006;22:851–853. doi: 10.1021/la052882r. [DOI] [PubMed] [Google Scholar]
- 15.Shum P, Kim JM, Thompson DH. Phototriggering of liposomal drug delivery systems. Adv. Drug Delivery Rev. 2001;53:273–284. doi: 10.1016/s0169-409x(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZY, Smith BD. Synthesis and characterization of NVOC-DOPE, a caged photoactivatable derivative of dioleoylphosphatidylethanolamine. Bioconjugate Chem. 1999;10:1150–1152. doi: 10.1021/bc990087h. [DOI] [PubMed] [Google Scholar]
- 17.Kono K, Murakami T, Yoshida T, Haba Y, Kanaoka S, Takagishi T, Aoshima S. Temperature-sensitization of liposomes by use of thermosensitive block copolymers synthesized by living cationic polymerization. Bioconjugate Chem. 2005;16:1367–1374. doi: 10.1021/bc050004z. [DOI] [PubMed] [Google Scholar]
- 18.Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Delivery Rev. 53:305. doi: 10.1016/s0169-409x(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 19.Boomer JA, Inerowicz HD, Zhang ZY, Bergstrand N, Edwards K, Kim JM, Thompson DH. Acid-triggered release from sterically stabilized fusogenic liposomes via a hydrolytic DePEGylation strategy. Langmuir. 2003;19:6408–6415. [Google Scholar]
- 20.Anyarambhatla GR, Needham D. Enhancement of the phase transition permeability of DPPC liposomes by incorporation of MPPC: A new temperature-sensitive liposome for use with mild hyperthermia. J. Liposome Res. 1999;9:491–506. [Google Scholar]
- 21.Reisch MS. Ushering cosmetics to the right spots. Chem. Eng. News. 2007;85:15–21. [Google Scholar]
- 22.Langereis S, Keupp J, van Velthoven JLJ, II, de Roos IHC, Burdinski D, Pikkemaat JA, Grull H. A temperature-sensitive liposomal 1H CEST and 19F contrast agent for MR image-guided drug delivery. J. Am. Chem. Soc. 2009;131:1380–1381. doi: 10.1021/ja8087532. [DOI] [PubMed] [Google Scholar]
- 23.Margin RL, Weinstein JN. In: In Liposome Technology. 3rd ed. Gregoriadis G, editor. Vol. 3. CRC Press; Boca Raton, FL: 2006. pp. 137–155. [Google Scholar]
- 24.Yatvin MB, Weinstein JM, Dennis WH, Blumentahl R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 25.Kong G, Dewhirst MW. Hyperthermia and liposomes. Int. J. Hyperthermia. 1999;15:345–370. doi: 10.1080/026567399285558. [DOI] [PubMed] [Google Scholar]
- 26.Lindner LH, Eichorn ME, Eibl H, Teichert N, Schmitt-Sody M, Issels RD, Dellian M. Novel temperature-sensitive liposomes with prolonged circulation time. Clin. Cancer. Res. 2004;10:2168–2178. doi: 10.1158/1078-0432.ccr-03-0035. [DOI] [PubMed] [Google Scholar]
- 27.Papahadjopoulos D, Jacobson K, Nir S, Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta. 1973;311:330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen W-H, Regen SL. Thermally gated liposomes. J. Am. Chem. Soc. 2005;127:6538–6539. doi: 10.1021/ja0513584. [DOI] [PubMed] [Google Scholar]
- 29.Otto S, Osifchin M, Regen SL. Squeezing a synthetic ionophore and mechanistic insight out of a lipid bilayer. J. Am. Chem. Soc. 1999;121:10440–1014. [Google Scholar]
- 30.Chen W-H, Shao X-B, Regen SL. Poly(choloyl)-based amphiphiles as pore-forming agents. Transport-active monomers by design. J. Am. Chem. Soc. 2005;127:12727–12735. doi: 10.1021/ja053527q. [DOI] [PubMed] [Google Scholar]
- 31.Bandyopadhyay P, Janout V, Zhang L, Regen SL. Ion conductors derived from cholic acid and spermine: importance of facial hydrophilicity on Na+ transport and membrane selectivity. J. Am. Chem. Soc. 2001;123:7691–7696. doi: 10.1021/ja010926m. [DOI] [PubMed] [Google Scholar]
- 32.Brocca P, Cantu L, Corti M, Del Favero E, Motta S, Nodari MC. Curved single-bilayers in the region of the anomalous swelling: effect of curvature and chain length. Colloids and Surfaces A: Physicochem. Eng. Aspects. 2006;291:63–68. [Google Scholar]
- 33.Kunneke S, Kruger D, Janshoff A. Scrutiny of the failure of lipid membranes as a function of headgroups, chain length and lamellarity measured by scanning force microscopy. Biophys. J. 2004;86:1545–1553. doi: 10.1016/S0006-3495(04)74222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ceppi P, Colombo S, Francolini M, Raimondo F, Borgese N, Masserini M. Two tail-anchored protein variants, differing in transmembrane domain length and intracellular sorting, interact differently with lipids. Proc. Natl. Acad. Sci. USA. 2005;102:16269–16274. doi: 10.1073/pnas.0508157102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.