Abstract
Structure-based drug design is underway to inhibit the S100B-p53 interaction as a strategy for treating malignant melanoma. X-ray crystallography was used here to characterize an interaction between Ca2+-S100B and a target, TRTK-12, which binds to the p53 binding site on S100B. The structures of Ca2+-S100B (1.5 Å resolution) and S100B-Ca2+-TRTK12 (2.0 Å resolution) determined here indicate that the S100B-Ca2+-TRTK12 complex is dominated by an interaction between Trp-7 of TRTK-12 and a hydrophobic binding pocket exposed on Ca2+-S100B involving residues in helices 2 & 3 and loop 2. As with a S100B-Ca2+-p53 peptide complex, TRTK-12 binding to Ca2+-S100B was found to increase the proteins Ca2+ ion binding affinity. One explanation for this effect was that peptide binding introduced a structural change that increased the number of Ca2+ ligands and/or improved Ca2+ ion coordination geometry of S100B. This possibility was ruled out when the structures of S100B-Ca2+-TRTK12 and S100B-Ca2+ were compared and calcium ion coordination by the protein was found to be nearly identical in both EF-hand calcium-binding domains (RMSD=0.19). On the other hand, B-factors for residues in EF2 of Ca2+-S100B were found to be significantly lowered with TRTK-12 bound. This result is consistent with NMR 15N relaxation studies that showed that TRTK-12 binding eliminated dynamic properties observed in Ca2+-S100B. Such a loss of protein motion may also provide an explanation for how calcium ion binding affinity is increased upon binding a target. Lastly, it follows that any small molecule inhibitor bound to Ca2+-S100B would also have to cause an increase in calcium ion binding affinity to be effective therapeutically inside a cell, so these data need to be considered in future drug-design studies involving S100B.
Keywords: S100B, TRTK-12, calcium, X-ray crystallography, NMR
Introduction
The small (10.7 kDa) acidic Ca2+-binding protein S100B is a member of the S100 family of proteins that were originally named because of their solubility in 100% ammonium sulfate1. S100 proteins have no intrinsic enzymatic activity, but like calmodulin (CaM) and most other EF-hand containing proteins, S100 proteins typically function as calcium-activated switches that bind to and regulate the biological function of numerous protein targets2; 3. For S100B, several of these protein targets include cytoskeletal and filament associated proteins (e.g. tubulin, GFAP, tau, desmin, vimentin, CapZ, calponin, calpactin I, and caldesmon), other Ca2+-binding proteins (e.g. annexins II, V, VI, S100A1, S100A6, S100A11, and neurocalcin-δ), membrane associated proteins (e.g. neuromodulin, neurogranin, MARCKS, giant protein AHNAK, and IQGAP1), transcription factors and their regulators (e.g. p53, hdm4, and hdm2) and several enzymes (e.g. aldolase, phosphoglucomutase, photoreceptor guanyl cyclase, Ndr kinase, and protein kinase C)4; 5.
There are several high resolution 3D structures of S100B available that are useful for understanding the calcium-dependent conformational change in S100B, which is necessary for its interaction with protein targets, including p53 and CapZ6; 7; 8; 9; 10; 11; 12; 13. In all of these structures, S100B is a symmetric dimer with a large number of hydrophobic interactions at its dimer interface consistent with its very high stability (KD < 1 nM)14; 15. In addition, each S100B subunit has two EF-hand calcium-binding domains that are brought into close proximity by a small two-stranded anti-parallel β-sheet. The second EF-hand (EF2; residues 61–72) has the consensus 12 amino acid sequence that represents a “typical” EF-hand16; 17, and binds calcium more tightly than the N-terminal EF-hand (EF1; residues 18–31). The pseudo-hand or S100-hand, EF1, has 14 rather than 12 residues and several of the ligands to calcium are from backbone carbonyl oxygen atoms rather than from sidechain carboxylate oxygen16; 17; 18; 19. A central loop (loop 2; residues 40–50) connects the two calcium-binding domains and is usually referred to as the “hinge region” of S100 proteins. It is this hinge and the C-terminal loops that are not conserved among the S100 protein family members, and it is these regions of the protein that contribute to specificity for interactions with various target proteins20.
A comparison of 3D structures of apo- and Ca2+-bound S100B illustrates a large rotation (~90°) in the position of helix 3 upon Ca2+-binding, exposing a hydrophobic cleft that is responsible for binding protein targets such as p53 and CapZ5; 6; 7; 11; 12; 13. When a peptide derived from the negative regulatory domain of p53 (367–388p53 peptide) binds to S100B, the affinity for Ca2+ in the typical EF-hand (EF2) of S100B was found to increase by 3-fold21. Consistent with this result, rates of Ca2+-dissociation measured in stopped-flow competition studies with Tb3+ indicate that the off-rate of Ca2+ is decreased by more than 8-fold in the presence versus the absence of bound p53367–388 22. Furthermore, mutation of S100B in the EF-hand calcium-binding domains (E72A or E31A) cause the proteins to exhibit significant broadening of NMR resonances in the presence of calcium, indicative of exchange on the chemical shift timescale, and such broadening is eliminated upon binding the p53 peptide. A similar result was also observed in this study when the TRTK-12 peptide binds to the E72A mutant of S100B. With these data in mind, it was proposed that target peptide binding to S100B-Ca2+ was sufficient to stabilize dynamic properties observed in S100B resulting in the overall tightening of calcium-ion binding10; 21; 23. However, an alternative explanation is that peptide binding to S100B-Ca2+ caused a conformational change in the EF-hand calcium-binding domain that introduced a new liganding residue and/or a more optimal Ca2+ coordination geometry. To test this alternate possibility, we have determined and directly compared crystal structures of S100B-Ca2+ and S100B-Ca2+-TRTK12. The structure of S100B-Ca2+-TRTK12 represents the first X-ray structure of S100B-Ca2+ bound to a target. These two structures also provide direct evidence that the coordination geometry for both EF-hand calcium-binding domains in S100B-Ca2+ are nearly identical in the absence and presence of bound TRTK-12 peptide and rule out the hypothesis that peptide binding induces a conformational change that increases the affinity of EF2 for calcium. Furthermore, the B-factors for several residues in the canonical EF-hand calcium binding site were significantly lower when TRTK-12 was bound to S100B-Ca2+ in the typical EF-hand (residues 61–72) and is consistent with previously reported NMR 15N relaxation studies of S100B-Ca2+ 24. Thus, the possibility that protein dynamics contribute to weaker calcium binding for S100B-Ca2+ in the absence of bound peptide target is discussed, as was suggested previously23; 24. These data are also relevant to studies aimed at developing inhibitors of S100B since any small molecule inhibitor (SBiX; S100B inhibitor) would not only have to bind S100B with high affinity, but it would also need to tighten calcium-binding upon complex formation, as was shown here for TRTK12, to have an effect in vivo.
Results
Metal ion binding to S100B in the absence and presence of the target peptide TRTK-12
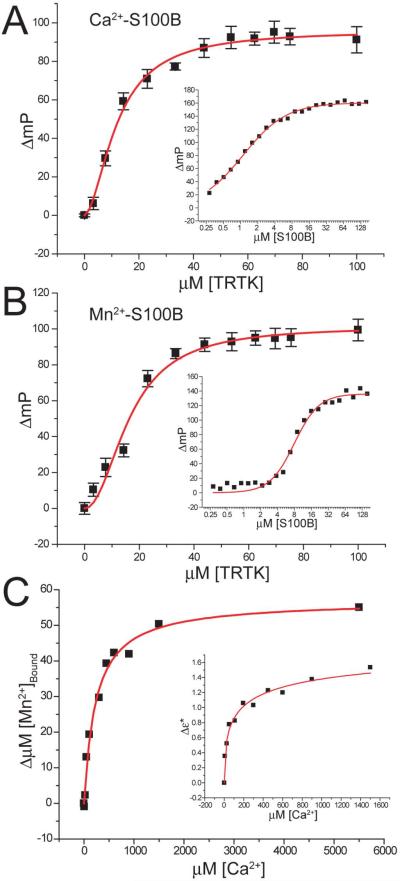
As described previously for the S100B-Mn2+-p53367–388 and S100B-Ca2+-p53367–388 complexes21; 23, metal ion binding to S100B was performed in the absence and presence of the peptide target, TRTK-12, to determine whether a peptide target other than p53367–388 can increase the affinity of S100B for metal ions (Mn2+ and Ca2+). Binding studies with Mn2+ were completed since it is a good probe of the high affinity Ca2+ binding site on S100B (EF2), as previously shown21; 25. The dissociation of TAMRA-TRTK12 from the S100B-Mn2+ and S100B-Ca2+ complexes were measured first to verify that TAMRA-TRTK12 could bind to S100B-Ca2+ and S100B-Mn2+. For these studies, a solution containing S100B-Ca2+ or S100B-Mn2+ was titrated into a solution of TAMRA-TRTK12 with Ca2+ or Mn2+ present, and changes in TAMRA fluorescence polarization from the TAMRA-TRTK12 peptide were monitored (Figure 1A and 1B; insets). Data from these titrations were fit to a single hyperbolic curve consistent with a single TAMRA-TRTK12 binding site per S100B subunit and dissociation constants (KD) of 6 ± 2 and 1.2 ± 0.2 μM for S100B-Mn2+ and S100B-Ca2+, respectively (Table 1). Unlabeled TRTK-12 was added next in competition binding studies to determine the dissociation constants of unlabeled TRTK-12 from each of the S100B-metal-TRTK12 complexes. These data were fit to a single hyperbolic curve and dissociation constants were calculated using the modified Cheng-Prusoff equation26. It was found that TRTK-12 bound to S100B-Mn2+ with a similar affinity (KD= 3.0 ± 1.0 μM; Table 1) as for S100B-Ca2+ (KD= 2.9 ± 0.5 μM; Table 1) and provided additional evidence that Mn2+ is a good probe for the study of EF-hand calcium signaling proteins, such as S100B. The formation of a S100B-Mn2+-TRTK12 complex was confirmed in a separate titration and was in agreement with titrations monitoring fluorescent polarization (Table 1); in which a solution of TRTK-12 plus Mn2+ was added to S100B-Mn2+ and a decrease in enhancement (ε*) of 1/T1P of water protons was observed using NMR21; 27. Such a deenhancement of the paramagnetic effect upon TRTK-12 binding is in agreement with structural studies that showed no direct coordination of TRTK-12 to Mn2+ in the ternary complex28.
Figure 1.
Thermodynamic binding constants for S100B-metal and S100B-metal-TRTK12 complexes. (a) Displacement of the TAMRA-TRTK12 peptide from the S100B-Ca2+-TAMRA-TRTK12 complex by unlabeled TRTK12 as monitored by fluorescence polarization. The solution contained 1.5 μM S100B, 50 nM TAMRA-TRTK12, and 10 mM CaCl2 in 50mM Tris-HCl pH 7.5. (a, inset) Binding of TAMRA-TRTK12 to S100B-Ca2+ as monitored by fluorescence polarization. The solution contained 50 nM TAMRA-TRTK12, and 10 mM CaCl2 in 50 mM Tris-HCl, pH 7.5. (b) Displacement of the TAMRA-TRTK12 peptide from the S100B-Mn2+-TAMRA-TRTK12 complex by unlabeled TRTK12 as monitored by changes in fluorescence polarization (ΔmP). The solution contained 4 μM S100B, 50 nM TAMRA-TRTK12, and 10 mM MnCl2 in 50mM Tris-HCl pH 7.5. (b, inset) Binding of TAMRA-TRTK12 to S100B-Mn2+ as monitored by changes in fluorescence polarization. The solution contained 50 nM TAMRA-TRTK12, and 10 mM MnCl2 in 50 mM Tris-HCl pH 7.5. (c) Displacement of bound Mn2+ by Ca2+ from S100B in the presence of TRTK as detected by EPR and NMR (inset). The solutions for both experiments contain 65 μM S100B, 150 μM TRTK-12, and 82 μM MnCl2 in 50mM Tris-HCl pH 7.5. The data were fit with the Hill equation to determine Kapp with the corresponding dissociation constants calculated using competition equations described in Material and Methods.
Table 1.
Dissociation of Metal Ions or Peptides from S100B-Metal or S100B-Metal-Peptide Complexesa
| Metal Ions | KD (μM)b | p53K2 (μM)c | TRTKK2 (μM)d | p53K3 (μM)e | TRTKK3 (μM)g | Tamara-TRTKK3 (μM)h |
|---|---|---|---|---|---|---|
| Ca2+ | 56 ± 9 (2)f | 20 ± 3 (5)f | 12 ± 10 (5) | 24 ± 7 (3)f | 2.9 ± 0.5 (2) | 1.2 ± 0.2 (2) |
| Mn2+ | 71 ± 12 (2)f | 27 ± 4 (2)f | 6.0 ± 2.0 (3) | 94 ± 20 (4)f | 3.0 ± 1.0 (3) | 6.0 ± 2.0 (2) |
The number of experiments (n) is shown in parentheses.
Dissociation constants of metals from the tight site (EF2) of S100B.
Dissociation constants (p53K2) of metals from the tight site of S100B (EF2) in the presence of p53 peptide.
Dissociation constants (TRTKK2) of metals from the tight site of S100B (EF2) in the presence of TRTK peptide.
Dissociation constants of p53 (p53K3) peptide from S100B-metal complexes.
Values reported in Rustandi et al, 1998.
Dissociation constants (TRTKK3) of TRTK-12 from S100B-metal complexes as determined by performing competition experiments with TAMARA-TRTK12.
Dissociation constants (Tamara-TRTKK3) of TAMARA-TRTK from metal-S100B complexes as measured directly by changes in fluorescence polarization.
The dissociation of Mn2+ from the S100B-Mn2+ and S100B-Mn2+-TRTK12 complexes was compared next using electron paramagnetic resonance (EPR; Supplementary Figure 1S). Under otherwise identical conditions (i.e. [S100B], [Mn2+], buffer, etc), the addition of stoichiometric amounts of TRTK-12 to a solution of S100B-Mn2+ significantly reduced the amount of free Mn2+ as measured by EPR (Fig. 1S). Thus, it was confirmed in a titration that the dissociation constant of Mn2+ from the S100B-Mn2+ complex (KD = 71±12 μM), as reported previously29, was 12-fold weaker than that of the dissociation of Mn2+ from the S100B-Mn2+-TRTK12 complex (KD = 6 ± 2 μM; Table 1). Such a result was not unexpected since the binding of a peptide derived from p53 (residues 367–388; p53367–388) also increased Mn2+ binding affinity; however, in the case of the S100B-Mn2+-p53367–388 complex, peptide binding only increased Mn2+ binding by 3-fold (Table 1)21.
Displacement of Mn2+ by Ca2+ in the S100B-Mn2+-TRTK12 complex was then achieved in competition studies to obtain the dissociation constant of Ca2+ from the S100B-Ca2+-TRTK12 complex (Figure 1C; Table 1). Specifically, the appearance of free Mn2+ was monitored by EPR upon the addition of Ca2+ (Figure 1C), and the data were confirmed by measurements of changes in enhancement (ε*) using NMR with the identical samples (Figure 1C, inset). In the first set of titrations, it was confirmed that Ca2+ was readily able to displace Mn2+ from the tight site (EF2) of S100B in the absence of TRTK-12, as found previously21. A competition experiment between Ca2+ and Mn2+ for S100B was performed next in the presence of TRTK-12 peptide (CaK2 = 12 ± 10 μM; Table 1), and bound TRTK-12 was found to enhance the affinity of Ca2+ by 5-fold in the S100B-Ca2+-TRTK12 complex versus in the absence of peptide (Table 1). Thus, as was found previously for S100B-metal ion complexes with the p53367–388 peptide21, the dissociation constants of Mn2+ and Ca2+ from the S100B-Mn2+-TRTK12 and S100B-Ca2+-TRTK12 complexes, respectively, were of higher affinity when compared to the analogous complexes in the absence the TRTK-12 target (Table 1). We next examined both the S100B-Ca2+ and the S100B-Ca2+-TRTK12 structures by X-ray crystallography to examine whether structural changes at or nearby the calcium-binding site(s) were responsible for the increased Ca2+-binding affinity observed when TRTK-12 is bound.
The X-ray structure of Ca2+-loaded S100B
For direct comparison, it was necessary to first solve the structure of S100B-Ca2+ in a manner similar to that used for the structure determination of S100B-Ca2+-TRTK12 (i.e. using the same cryoprotection, collection, and refinement techniques), so the structural differences and the B-factors of the two structures could be most accurately compared. With this in mind, the 1.50 Å resolution structure for S100B-Ca2+ was solved and compared to the S100B-Ca2+-TRTK12 (see below). The final asymmetric unit of Ca2+-bound S100B consisted of two S100B subunits (model A and model B) as a symmetric dimer with each subunit consisting of 88-residues for S100B (Ser1 to Phe88), two calcium ions per S100B subunit and a total of 146 water molecules. Nearly all of the residues of the S100B-Ca2+ were in the most favorable region of the Ramachandran plot (94.5%) with the remaining residues in the additionally allowed region (5.5%) (Table 2; Figure 2A). The global fold of S100B-Ca2+ was nearly identical to that of the X-ray structure reported previously (RMSDall atoms of ~0.26 for both models A and B) with all of the Ca2+-ligands, ligand distances, helical angles, and EF-hand angles found to be very similar (Tables 3–5). Specifically, each subunit of S100B-Ca2+ contained four helices (helix 1, S1-G19; helix 2, K28-L40; helix 3, E49-D61; helix 4, D69-F88) with the dimer interface aligned as a symmetric X-type four helix bundle and two helix-loop-helix EF-hand calcium-binding domains including an “S100-type” or “pseudo” EF-hand comprising helices 1, 2 and loop 1, and a “typical” EF-hand with twelve residues contributed by helices 3, 4 and loop 3 (Figure 2). Furthermore, the refined NMR structure of S100B-Ca2+ was also very similar to the X-ray structures of calcium-bound S100B24. The only difference observed was with the length of helix 4, which was well defined up to residue Ala-83 in the NMR structure (versus Phe-88 in the X-ray structure). The remaining residues in the C-terminus (C84-E91) of calcium-bound S100B were found to exhibit a significant amount of conformational exchange and fast-time scale motion as a result of its dynamic nature in solution10; 12; 24; 30; 31; 32. In the X-ray crystal structures, it is likely that this C-terminal loop of S100B is stabilized by crystal lattice contacts involving residues at the C-terminus including Phe-87 and Phe-88 of calcium-bound S100B resulting in the stabilization of helix 4 out to Phe-88.
Table 2.
X-ray Diffraction and Model Refinement Statistics
| Ca2+-S100Ba | TRTK-Ca2+-S100B | |
|---|---|---|
| Diffraction Statistics | ||
| Space Group | C21 | C2221 |
| Cell dimensions a, b, c (Å) | 89.6, 35.0, 58.1 | 33.0, 89.8, 58.9 |
| Cell angles α, β, γ (deg) | 90, 92.6, 90 | 90, 90, 90 |
| Resolution (Å) | 58.03–1.50 (1.54–1.50)b | 44.90–2.01 (2.06–2.01)a |
| No. of unique reflections | 24746 (1408) | 5763 (421) |
| Completeness (%) | 89.1 (68.4) | 98.7 (97.8) |
| Rsymc | 0.061 (0.247) | 0.066 (0.200) |
| Average I/σ | 13.9 (2.8) | 28.8 (13.4) |
| Multiplicity | 2.6 (1.5) | 12.7 (12.9) |
| Refinement Statistics | ||
| Rcrysd (%) | 20.2 (23.8) | 22.3 (22.1) |
| Rfreed (%) | 24.2 (35.6) | 26.7 (27.2) |
| Protein Atoms | 1452 | 796 |
| Water Molecules | 146 | 33 |
| Non-Hydrogen Atoms | 1599 | 832 |
| RMSD | ||
| Bond Length (Å) | 0.015 | 0.014 |
| Bond Angles (Å) | 1.333 | 1.736 |
| Mean B values (Å2) | 32.56 | 34.61 |
| Ramachandran plot (%)e | ||
| Most Favored | 94.5 | 93.3 |
| Additionally Allowed | 5.5 | 5.6 |
| Generously Allowed | 0.0 | 1.1 |
| PDB identification | 3IQO | 3IQQ |
The previously publish Ca2+-S100B (1MHO) was compared to Model A and Model B of the Ca2+-S100B structure represented here, the RMSD changes were very minimal at 0.251 and 0.266, respectively.
Numbers in parentheses represent the last outer shell.
Rsym = ΣhΣi(|li(h)| - |{l(h)}|)/ΣhΣilj(h), where li(h) = observed intensity, and {l(h)} = mean intensity obtained from multiple measurements.
Rcrys and Rfree = Σ||Fo| - |Fc||/Σ||Fo|, where |Fo| = observed structure factor amplitude and |Fc| = calculated structure factor amplitude for the working and test sets, respectively.
For Ca2+-S100B the calculations had 155 residues in the most favored region and 9 residues in additionally allowed regions and for TRTK-Ca2+-S100B the calculations had 84 residues in the most favored region, 5 residues in additionally allowed regions, and 1 residue in the generously allowed regions.
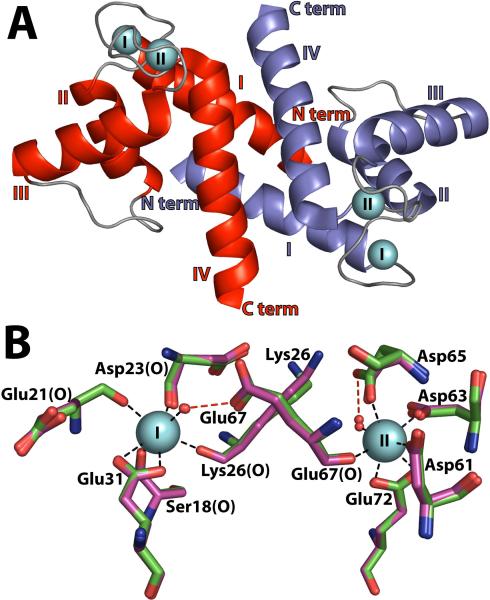
Figure 2.
Calcium ion coordination in the X-ray structures of S100B-Ca2+ in the presence and absence of TRTK-12. (a) X-ray crystal structure of S100B-Ca2+ (3IQO) shown in a ribbon diagram with two Ca2+ ions per subunit (cyan spheres) labeled I (EF1, Pseudo EF-hand) and II (EF2, Typical EF-hand) with the subunits of the symmetric S100B homodimer colored red and blue with alpha helices labeled (I–IV). (b) The positions for the sidechains of the Ca2+ coordinating residues are compared for the pseudo-EF-hand (Ser18, Glu21, Asp23, Lys26 and Glu31) and the canonical EF-hand (Asp61, Asp63, Asp65, Glu67, and Glu72) calcium-binding sites of S100B-Ca2+ (green) and S100B-Ca2+-TRTK12 (Magenta) (RMSD = 0.18 Å).
Table 3.
| Ca2+-S100B (A) | Ca2+-S100B (B) | TRTK-Ca2+-S100B | |
|---|---|---|---|
| Pseudo EF Hand (EFI) | |||
| Ser-18 (C=O) | 2.3 | 2.4 | 2.3 |
| Glu-21 (C=O) | 2.4 | 2.4 | 2.3 |
| Asp-23 (C=O) | 2.3 | 2.3 | 2.4 |
| Lys-26 (C=O) | 2.4 | 2.4 | 2.4 |
| Glu-31 (Oε1, Oε2) | 2.5, 2.6 | 2.4, 2.6 | 2.4, 2.7 |
| H2Od | 2.6 | 2.3 | 2.3 |
| Canonical EF Hand (EF2) | |||
| Asp-61 (Oδ1 or Oδ2) | 2.3 | 2.3 | 2.2 |
| Asp-63 (Oδ1 or Oδ2) | 2.3 | 2.3 | 2.4 |
| Asp-65 (Oδ1 or Oδ2) | 2.5 | 2.4 | 2.4 |
| Glu-67 (C=O) | 2.3 | 2.3 | 2.3 |
| Glu-72 (Oε1, Oε2) | 2.4, 2.6 | 2.4, 2.6 | 2.5, 2.7 |
| H2Oe | 2.4 | 2.4 | 2.4 |
| Ca2+-bound H2O | |||
| Asp-65 (Oδ1 or Oδ2)c | 2.6 | 2.8 | 2.8 |
| Glu-67 (Oε1 or Oε2)d | 2.6 | 2.7 | 2.5 |
All distances are in Å and are from Nitrogen, Oxygen, or Ca2+.
Distances between the two Ca2+ ions in EF1 and EF2 for the Ca2+-S100B (A), Ca2+-S100B (B), and the TRTK-Ca2+-S100B structures are all 11.4 Å.
Distances for the H2O ligands to the Ca2+ ions in the pseudo EF Hand (EF1).
Distances for the H2O ligands to the Ca2+ ions in the canonical EF Hand (EF2).
Table 5.
| N-terminal coordinate of second helix | Θ (°) | Φ (°) | Ω (°) | |||
|---|---|---|---|---|---|---|
| Pseudo EF hand | ||||||
| Ca2+-S100B (X-ray)c | 8.2 | −0.1 | −6.8 | 50.0 | 86.8 | 109 |
| Ca2+-S100B (A; X-ray) | 8.3 | 0.0 | −6.7 | 48.6 | 85.4 | 110 |
| Ca2+-S100B (B; X-ray) | 8.2 | −0.1 | −6.7 | 50.0 | 85.7 | 110 |
| TRTK-Ca2+-S100B (X-ray) | 8.1 | 0 | −6.6 | 48.3 | 85.4 | 111 |
| Ca2+-S100B (NMR)d | 10.4 | −3.2 | −7.9 | 48.6 | 75.8 | 120 |
| TRTK-Ca2+-S100B (Inman et al.)e | 9.4 | −1.3 | −7.5 | 54.4 | 93.3 | 103 |
| TRTK-Ca2+-S100B (Shaw et al.)f | 10.3 | 0.1 | −4.1 | 52.3 | 101.4 | 128 |
| Typical EF hand | ||||||
| Ca2+-S100B (X-ray)a | 8.9 | 0.3 | −6.6 | 74.3 | 101.9 | 75 |
| Ca2+-S100B (A; X-ray) | 8.7 | 0.3 | −6.9 | 74.4 | 102.0 | 73 |
| Ca2+-S100B (B; X-ray) | 8.7 | 0.3 | −6.8 | 75.0 | 102.0 | 73 |
| TRTK-Ca2+-S100B (X-ray) | 8.8 | 0.5 | −6.6 | 74.3 | 102.0 | 75 |
| Ca2+-S100B (NMR)d | 8.9 | −2.6 | −8.3 | 71.3 | 82.5 | 94 |
| TRTK-Ca2+-S100B (Inman et al.)e | 8.6 | 1.8 | −5.6 | 67.0 | 116.2 | 67 |
| TRTK-Ca2+-S100B (Shaw et al.)f | 8.8 | −2.7 | −4.6 | 75.5 | 91.3 | 113 |
Vector Geometry Mapping (VGM) angles were calculated using VGM software (K. Yap, University of Toronto) with the helices and structures as indicated.
The residues used for the VGM calculations were as follows: helix I, residues 10–17; helix II, residues 29–39; helix III, residues 53–60; and helix IV, residues 70–80.
Calculated using PDB entry 1MHO.
Calculated using PDB entry 2K7O.
Calculated using PDB entry 1MWN.
Calculated using PDB entry 1MQ1.
The X-ray structure of S100B-Ca2+-TRTK12 complex
The structure of S100B-Ca2+-TRTK12 represents the first X-ray crystal structure of an S100B-Ca2+-target complex, so it can be used to answer an important unresolved question about whether or not structural changes resulting from target protein binding directly affect Ca2+ ion coordination in either of the two EF-hand calcium binding domains of S100B. If such a change in calcium ion coordination occurred, then this could provide an explanation for how divalent metal ions bind to S100B with a higher affinity when a target peptide is bound (Figure 1; Table 1). This question could not be answered conclusively via the existing NMR structures of S100B-Ca2+-target peptide complexes since the position of the bound Ca2+ ions could not be measured directly by NMR10; 31; 33; 34. Furthermore, the X-ray structure of S100B-Ca2+-TRTK12 determined here will also be useful for resolving a discrepancy between two existing NMR structures31; 33; 34, with regard to the helical conformation of the bound TRTK-12 peptide. Lastly, this structure will also be particularly useful for structure-based drug design studies aimed at inhibiting the S100B-p53 interaction in malignant melanoma since the S100B-TRTK-12 complex directly inhibits full-length p53 from binding Ca2+-S100B35.
With these issues in mind, the 2.01 Å resolution X-ray structure for S100B-Ca2+-TRTK12 was solved. The final asymmetric unit consisted of 89-residues for S100B (Met0 to Phe88), 9-residues for TRTK-12 (Thr3 to Leu 11), two calcium ions, and 33 water molecules. The biologically significant model is a dimer comprised of the asymmetric unit and a crystallographic symmetry mate. Nearly all of the residues of the S100B-Ca2+-TRTK12 were in the most favorable region of the Ramachandran plot (93.3%) with the remaining residues in the additionally (5.6%) and generously (1.1%) allowed region (Table 2; Figure 3B). Unlike what was found for S100B-Ca2+ in solution, the NMR and X-ray structures of S100B-Ca2+-TRTK12 were in agreement with regard to the length of helix 4, which extended out to residue Phe-87 both in solution and in the crystalline form. This extended helical region was stabilized by direct interactions with the TRTK-12 peptide including hydrophobic interactions between Phe-87 in S100B and hydrophobic residues in the peptide as well as by a hydrogen bond between the backbone carbonyl oxygen of Glu-86 of S100B and the sidechain of Lys-9 in TRTK-12 (Figure 4).
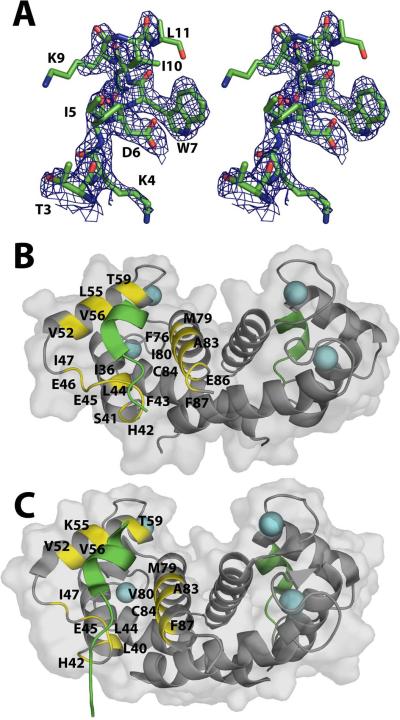
Figure 3.
The X-ray and NMR structures of the S100B-Ca2+-TRTK12 complex. (a) Stereo view (walleye mode) of the TRTK-12 peptide when bound to S100B with the electron density maps calculated with the 2mFo-DFc coefficients (1.0σ) for TRTK-12. (b) Ribbon and surface diagram of the X-ray structure of S100B-Ca2+-TRTK12 (3IQQ) illustrating the location of TRTK-12 (green) together with residues of S100B that interact with the peptide (in yellow). (c) Ribbon and surface diagram of the NMR structure of S100B-Ca2+-TRTK12 (1MWN) illustrating the location of TRTK-12 (green) bound to rat S100B with residues colored (in yellow) that have intermolecular NOE correlations to TRTK-1233.
Figure 4.
Side-chains of S100B and TRTK-12 that are involved in the peptide-protein interface. (a) TRTK-12 peptide residues that are observed in the X-ray crystal structure are illustrated (T3-L11) highlighting residues on S100B that are within 4.0 Å of TRTK peptide (in boxes) and residues that are involved in hydrogen bonding in red. (b) View of TRTK-12 (green) and S100B (blue) illustrating side-chains involved in hydrophobic interactions (yellow) between the TRTK-12 and S100B. (c) View of TRTK-12 (green) and S100B (blue) illustrating side-chains involved in hydrogen bonds (dashed lines) between TRTK-12 and S100B. (d) The X-ray structure of the bovine S100B-Ca2+-TRTK12 complex (shown in blue and green) superposed on the NMR solution structure of the same complex with rat S100B (red PDB: 1MWN).
Also consistent with both NMR structures31; 33, the X-ray structure of S100B-Ca2+-TRTK12 illustrates that one TRTK-12 peptide binds per S100B subunit. The protein-peptide interaction of TRTK-12 with S100B is mostly hydrophobic with several hydrogen bonds, giving rise to a relatively high affinity interaction when compared to other peptide-protein interactions with S100B4; 36. Of the total TRTK-12 solvent-accessible area 48.1% (586.7/1219.3 Å2) is involved in the S100B-Ca2+/peptide interface, and the TRTK-12 peptide occupies only 8.1% of the total solvent-accessible area of each S100B subunit (497.6/6160.7 Å2). The hydrophobic residues Ile-5, Trp-7, Ile-10 and Leu-11 of TRTK-12 contribute most of the buried surface area in the interaction interface. Residues of S100B contributing to hydrophobic peptide interactions are found on helix 2 (I36), loop 2 termed the `hinge' (H42, F43, L44, E45, I47), helix 3 (V52, K55, V56, T59) and helix 4 (F76, M79, I80, A83, C84, E86, F87). The hinge of S100B connects helices 2 and 3 with residues from this loop including His-42, Phe-43, and Glu-45 found to contribute H-bonds to Thr-3, Lys-4, Ile-5, Asp-6, Trp-7 and Lys-9 of TRTK-12 (Figure 4). This specific interaction between the peptide and the protein created two alpha-helical turns forming hydrogen bonds on the TRTK-12 between Asp-6(O)-Lys-9(N) (3.54 Å) and Trp-7(O)-Leu-11(N) (2.52 Å). The helical nature of the peptide is representative of the helical nature that is found in the crystal structure of the capZ protein (PDB: 1IZN), indicating that TRTK-12 is in its most stable conformation. Furthermore, this helical structure very closely matches that found in solution as reported for one of the previously published NMR structures33 (Figure 4D).
Comparisons of the structures of S100B-Ca2+-TRTK12 and S100B-Ca2+
Upon the addition of calcium to dimeric apo-S100B, helix 3 of both subunits reorients by ~90° relative to helix 4 as is found for most S100 proteins3; 5; 6; 8; 11; 12; 21; 24; 30; 31; 33. This conformational change exposes residues on loop 2 termed the `hinge' (residues H42, F43, L44, E45, E46, I47), helix-3 (V51, K55, V56, T59), and helix-4 (F76, M79, I80, A83, C84, F87) and provides the “open” Ca2+-bound form of S100B, which binds target peptides such as TRTK-12 (Figures 2–4). To determine the structural consequences of binding the target peptide, TRTK-12, we determined and compared the crystal structures of S100B-Ca2+ and S100B-Ca2+-TRTK12 (Figures 5 and 6). As a control, we first compared the two asymmetric units of Model A (subunit 1) and Model B (subunit 2) of the symmetric S100B-Ca2+ dimer for which very minimal changes were observed (overall mainchainRMSD = 0.2 Å; sidechainRMSD = 0.5 Å). These included differences in positioning of the mainchain for residues in the pseudo-EF-hand (E21, G22) and helix 4 (E86, F87, F88) and differences in sidechain positioning for residues in helix 1 (E2, K5, V13), the pseudo-EF-hand (E21, K24, H25, K28), helix 3 (E58) and helix 4 (Q71, T81, E86, F87, F88) (Figures 5A & 5B). Such differences between Model A and B are not surprising in a small protein such as S100B, and are likely the result of varying crystal packing environments, although it cannot be ruled out that these differences represent two conformations of nearly the same free energy. As expected, additional differences were observed when the structures of Ca2+-S100B (both models) and S100B-Ca2+-TRTK12 were examined side-by-side that were not observed when models A and B of Ca2+-S100B were compared (colored red in Figures 5C, 5D, 5E, & 5F only when the difference was observed for both models). Specifically, main chain residues that changed position when TRTK-12 bound to Ca2+-S100B (RMSD>0.4 Å; highlighted in red on Figs. 5C, 5E) included residues in helix 1 (S1, E2, L3, E4, K5, V8, A9), loop 2 (Q50, V53), and helix 4 (H85). Likewise, several sidechains changed positions (RMSD>1.0 Å; highlighted in red on Figs. 5D, 5F) in similar regions as the mainchain including residues in helix 1 (Q16), loop 2 (E45, Q50), and helix 4 (M79, H85). In conclusion, several regions of the protein were conclusively found to change position upon the addition of TRTK-12 to S100B-Ca2+ while many residues that have direct interaction with TRTK-12 did not change position. These side chain residues form hydrophobic contribution of TRTK-12 binding. TRTK-12 directly interacting with these hydrophobic residues may help stabilize the S100B in the “open” Ca2+ bound position.
Figure 5.
Changes in main and side-chain positioning for the two asymmetric units of S100B-Ca2+ (model A and B) upon binding TRTK-12. (a) The average RMSDs for the position of main-chain atoms for the two asymmetric units, Model A and Model B, were compared for the X-ray structure of S100B-Ca2+. (b) The average RMSDs for the position of side-chain atoms for the two asymmetric units, Model A and Model B, were compared next for the X-ray structure of S100B-Ca2+. (c) The average RMSDs for the position of main-chain atoms were compared for the X-ray structures of S100B-Ca2+ (model A) and S100B-Ca2+-TRTK12. (d) The average RMSDs for the position of side-chain atoms were compared for the X-ray structures S100B-Ca2+ (model A) and S100B-Ca2+-TRTK. (e) The average RMSDs for the position of main-chain atoms were compared for the X-ray structures of S100B-Ca2+ (model B) and S100B-Ca2+-TRTK12. (f) The average RMSDs for the position of side-chain atoms were compared for the X-ray structures of S100B-Ca2+ (model B) and S100B-Ca2+-TRTK. Those residues that have an average RMSD for main-chain and side-chain value greater than 0.4 and 1.0 Å, respectively, are labeled. Residues of S100B-Ca2+ that are affected by TRTK-12 binding are labeled in red.
Figure 6.
Graph showing B-factor values for each residue in S100B-Ca2+ in the absence and presence of bound TRTK-12. (a) Average of main-chain atoms B-factor values per residue of S100B for S100B-Ca2+; model A (blue diamonds), S100B-Ca2+; model B (green squares), S100B-Ca2+-TRTK (red triangle). (inset) Average of main-chain atoms B-factor values per residues of S100B for S100B-Ca2+-pentamidine (orange pluses) and S100B-Ca2+-TRTK (red triangle). (b) Average of all atoms B-factors per residue of S100B for S100B-Ca2+; model A (blue diamonds), Ca2+-S100B; model B (green squares), S100B-Ca2+-TRTK (red triangle). (inset) Average of all atoms B-factors per residue of S100B for S100B-Ca2+-pentamidine (orange pluses) and S100B-Ca2+-TRTK (red triangle). (c) Ribbon diagram of the X-ray structure of S100B-Ca2+-TRTK (3IQQ) illustrating the residues of S100B (in red) that showed significant changes in B-factor values when the X-ray structural models for S100B-Ca2+-TRTK and S100B-Ca2+ (i.e. for both models A and B) were compared.
Calcium-coordinating residues in S100B are the same and positioned nearly identically when the structures of S100B-Ca2+ and S100B-Ca2+-TRTK12 are compared
Although there were some changes in the conformation and orientation of specific residues of S100B-Ca2+ upon binding TRTK-12 (Figure 5), the residues that coordinate calcium in S100B-Ca2+-TRTK12 were found to be the same as those for S100B-Ca2+ with minimal differences in coordinate distances and positions (RMSD: 0.19 Å; Table 3; Figure 2B). Specifically, calcium coordination in the S100-hand of S100B-Ca2+ and S100B-Ca2+-TRTK12 was from four backbone carbonyl oxygen atoms including Ser-18 (X position), Glu-21 (Y position), Asp-23 (Z position), and Lys-26 (−Y position), a water molecule (−X position), and the two sidechain carboxylate oxygen atoms of Glu-31 (−Z position; bidentate ligand). In the typical EF-hand, calcium was coordinated by sidechain carboxylate oxygen atoms of Asp-61 (X position), Asp-63 (Y position), Asp-65 (Z position), the backbone carbonyl of Glu-67 (−Y position), a water molecule (−X position), and two sidechain carboxylate oxygen atoms of Glu-72 (−Z position; bidentate ligand) (Figure 2B). As discussed previously37, a side-chain carboxylate oxygen atom of Glu-67 formed a hydrogen bond with a water molecule coordinated to calcium at the −X position of the pseudo-EF hand in S100B-Ca2+-TRTK12 (Figure 2B, Table 3). Thus, it can be concluded that TRTK-12 binding did not affect calcium-coordination distances or the positioning of the residues involved in binding calcium in either EF1 or EF2 (Figure 2B).
Ca2+-binding is restored to the E72A mutant of S100B in the presence of TRTK-12 as determined by NMR spectroscopy
Wild-type S100B binds calcium in the typical EF-hand (residues 61–72; EF2 KD = 56 ± 9 μM; Table 1) more tightly than the pseudo EF-hand (residues 18–31; EF1 KD > 350 μM), and a conformational change occurs upon binding EF2, which is necessary to bind target proteins such as TRTK-128; 10; 30. One notable NMR chemical shift perturbation that occurs upon the addition of calcium to wild-type S100B is a 2.12 ppm downfield shift in the amide 1H for Gly-66. This residue is in position 6 of the 12 consensus residues in EF2, and the chemical shift perturbation for this glycine residue upon binding calcium is very typical for all EF-hand calcium-binding domains30. This and several other perturbations (>400 Hz) are slow on the chemical shift timescale and provide a limiting value for the rate of calcium ion dissociation (<400/sec). Furthermore, TRTK-12 peptide binding to Ca2+-S100B is found to enhance calcium binding in the typical EF-hand by 5-fold (Table 1), despite the fact that no TRTK-12 peptide binding occurs in the absence of calcium even at high protein and peptide concentrations (>3 mM). Therefore, not surprisingly, the chemical shift of Gly-66 is also found to be significantly downfield shifted in the S100B-Ca2+-TRTK12 complex, and this chemical shift value (1H, 10.11 ppm; 15N, 114.21 ppm) is only very slightly shifted from that found in Ca2+-S100B (1H, 10.13 ppm; 15N, 114.28 ppm).
To examine the calcium-dependent S100B-target protein interaction more closely, the EF-hand mutant, E72A, was examined next in the absence and presence of bound TRTK-12 via NMR spectroscopy. As previously observed, the chemical shift values for the apo-E72A mutant were minimally different from those reported previously for wild-type S100B16; 23, and the perturbations that were observed are for the most part localized to the general area of the mutation, which is indicative that no large structural rearrangement or problem with folding occurred as a result of the mutation. However, unlike what was observed previously in calcium ion titrations with wild-type S100B30, the addition of calcium severely broadens several of the 1H-15N correlations for the E72A mutant of S100B, including the HSQC correlation for Gly-66, which completely disappears. Furthermore, the spectra did not improve even at very high levels of calcium (20 mM). For Gly-66, this broadening effect is most readily explained by the weakened calcium-binding affinity of the E72A mutant protein giving rise to intermediate exchange on the chemical shift timescale or severe conformational exchange, as reported previously23. Whereas, for wild-type S100B, several residues, including Gly-66, disappear and then reappear later in the titration consistent with slow exchange kinetics on the chemical shift timescale30. For the E72A mutant, calcium binding could be measured, but it was consistent with only a single calcium ion binding to the psuedo EF hand of each S100B subunit (EF1 KD = 480 ± 130 μM)23. The fact that this residual calcium binding (EF1 KD = 480 ± 130 μM) was indeed due to the pseudo EF-hand was confirmed by observations of tyrosine sensitized Tb3+ luminescence from Tyr-17 to Tb3+, which occurs only when Tb3+ is bound to EF1 as is described in detail previously23; 38. Consistent with what was observed by NMR titrations, it was also found that binding of the TRTK-12 peptide to the E72A mutant is nearly identical to that found for the wild-type protein (KD = 3.2 ± 1.5 μM; Supplemental Fig. 2S). This binding occurred despite their large decreases in calcium binding in the absence of peptide. Furthermore, TRTK-12 binding to the E72A mutant of Ca2+-S100B eliminated much of the conformational exchange observed for the E72A-Ca2+ complex in the absence of peptide, and the appearance of the downfield shifted correlation for Gly-66, which typifies calcium binding in the slow-exchange regime to EF2, was restored (Fig. 2S; 1H=10.07 ppm; 15N=113.83 ppm). These results indicate that the presence of a target peptide, such as TRTK-12, restored the ability of the E72A mutant of S100B to undergo the calcium-dependent conformational change involving EF2 as is necessary to bind target peptide. The restoration of calcium binding to the E72A mutant of S100B was also observed previously in titrations with a peptide derived from the C-terminus of p5323, so this effect on calcium-binding is not specific for a single target peptide.
Discussion
In the absence of calcium, the TRTK-12 peptide does not bind to S100B, even at mM concentrations33. Thus, as with several target protein interactions involving S100B, the interaction between S100B and TRTK-12 is dependent upon the addition of calcium4; 5; 20; 21; 31; 33; 39; 40; 41; 42. As observed previously, most of the residues that interact with TRTK-12 (11 of 15; Fig. 4) are buried in the apo-S100B structure, but are exposed due to a large change in the position of helix 3 upon the addition of calcium explaining the calcium-dependent S100B-TRTK12 interaction6; 8; 11; 12; 13; 30; 31; 33; 43. Furthermore, the relative orientation of all four helices in both subunits of dimeric S100B in the S100B-Ca2+-TRTK12 structures are nearly identical when the X-ray structure determined here and the two NMR structures determined previously are compared (Table 4 and 5)31; 33. We also have found that a major component of the S100B-TRTK12 interface involves a hydrophobic interaction between the tryptophan residue of TRTK-12 (W7) and several hydrophobic residues from helices 3, 4, and loop 2 termed the `hinge' region of Ca2+-S100B (Figure 4). There is also agreement in the identity and relative position of several sidechains at the S100B-TRTK12 interface when the X-ray structure of bovine S100B determined here is compared to the NMR structure of rat S100B bound to TRTK-12. This is likely because both of these structures illustrate that TRTK-12 bound to S100B forms a very small helix (Figure 4D). Thus, in addition to interactions with Trp-7, several other hydrophobic residues of TRTK-12 at the peptide-protein binding interface are the same including Ile-5, Ile-10, and Leu-11. In contrast, in another NMR structure of the human S100B-TRTK12 complex, it was reported that the peptide is in an extended conformation with several of the sidechain moieties of TRTK-12 positioned differently. Nonetheless, in all three structures, the tryptophan residue of the TRTK-12 peptide, Trp-7, is at the center of the S100B-TRTK12 interaction and is proximal to nine hydrophobic residues of S100B (Figure 4), indicating that this residue is critically important for binding under all conditions. In fact, when a peptide derived from p53 is mutated to a tryptophan at the analogous position (F387W), the affinity for this peptide, p53367–388; F387W goes up by 3- to 4-fold21. Thus, the hydrophobic residues that define the S100B-TRTK12 interface include Ile-5, Trp-7, Ile-10, and Leu-11 of TRTK-12 and are extensively involved in a mini-hydrophobic core that likely determines the orientation of TRTK-12 peptide in the Ca2+-S100B binding pocket (Figure 4). While some ionic interactions and hydrogen bonds are also observed here (Figure 4C), they are fewer in number and probably do not contribute as much to the binding of TRTK-12, as discussed previously36.
Table 4.
Interhelical angles and distances for structures of S100B
| Interhelical Angles (°)a,b | Interhelical distance (Å)a,b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I to II | I to III | I to IV | II to III | II to IV | III to IV | I to II | I to III | I to IV | II to III | |
| Ca2+-S100B (X-ray)c | 140 | −121 | 125 | 99 | −32 | 110 | 15 | 22 | 12 | 12 |
| Ca2+-S100B-A(X-ray) | 143 | −120 | 126 | 97 | −32 | 109 | 14 | 22 | 12 | 12 |
| Ca2+-S100B-B(X-ray) | 143 | −120 | 127 | 98 | −31 | 109 | 14 | 22 | 13 | 12 |
| TRTK-Ca2+-S100B (X-ray) | 143 | −117 | 128 | 99 | −32 | 110 | 14 | 22 | 12 | 12 |
| Ca2+-S100B (NMR)d | 138 | −127 | 124 | 93 | −36 | 106 | 17 | 25 | 14 | 12 |
| TRTK-Ca2+-S100B (NMR; Inman et al)e | 132 | −116 | 122 | 108 | −35 | 119 | 15 | 22 | 12 | 11 |
| TRTK-Ca2+-S100B (NMR; Shaw et al)f | 134 | −105 | 132 | 115 | −48 | 111 | 15 | 21 | 13 | 12 |
Interhelical angles and distances were calculated using INTERHLX software (K. Yap, University of Toronto).
The residues defining each helix of S100B were as follows: helix I, residues 2–18; helix II, residues 29–40; helix III, residues 50–62; and helix IV, residues 70–82.
Calculated using PDB entry 1MHO.
Calculated using PDB entry 2K7O.
Calculated using PDB entry 1MWN.
Calculated using PDB entry 1MQ1.
More recently, S100B has gained attention because it binds directly to the p53 tumor suppressor protein in melanoma, reduces p53 protein levels, and inhibits wild-type p53 functions in malignant melanoma 44; 45. Therefore, several groups are working to develop inhibitors of S100B with the goal of restoring functional p53 into cancers such as malignant melanoma. Such structure-based drug designed approaches will benefit greatly by this new X-ray structure of S100B bound to TRTK-12 particularly because TRTK-12 directly inhibits full length p53 from binding to Ca2+-S100B 35. Specifically, methods in designing/identifying small molecule inhibitors of S100B should make use of the fact the Trp-7 of TRTK-12 binds a well-defined hydrophobic pocket of S100B and that the majority of the S100B-TRTK12 interactions are hydrophobic with only a few hydrogen bonds. Thus, it is now clear that any small molecule inhibitor of S100B would benefit by binding this same hydrophobic pocket on S100B as defined by this structure of S100B bound to TRTK-12.
In addition to defining the S100B-TRTK12 interface, another major purpose for solving the structure of S100B-Ca2+-TRTK12 was to evaluate whether any changes in calcium-ion coordination resulted in Ca2+-bound S100B upon binding the TRTK-12 peptide. This question is of importance because of the observation here and elsewhere that target peptide binding increases the affinity of S100B for calcium23. While some changes were observed in the positioning of specific residues in Ca2+-S100B upon binding TRTK-12 (Figure 5), the residues that coordinate calcium in S100B-Ca2+-TRTK12 do not move when compared to those for S100B-Ca2+ (RMSD: 0.19Å; Table 3; Figure 2B). Thus, TRTK-12 binding did not induce a conformational change in Ca2+-bound S100B that could explain the increase in calcium affinity observed (Table 1). A closer look at the structure reveals that there are some residues on S100B that form hydrophobic interactions with TRTK-12, which are nearby the typical EF hand. This includes residues on helix 3 (V56 and T59) and helix 4 (F76). For example, Val-56 and Phe-76 each form i,i+4 hydrogen bonds to residues Leu-60 and Glu-72, respectively. Leu-60 is shown to be directly below the EF-hand while Glu-72 is the bidentate coordinating residue of EF2. Thus, TRTK-12 binding, may stabilize the EF-hand and increase Ca2+ affinity for the typical EF-hand in S100B through secondary interactions and H-bonds.
We next examined whether temperature factors (or B-factors) differed between the crystal structures of S100B-Ca2+ and S100B-Ca2+-TRTK12. In such a comparison, the B-factors account for random displacements of atoms from their mean position as a result of either lattice disorder and/or from thermal motion; however, the timescale of such motions cannot be ascertained from a standard diffraction experiment. An interesting observation was made when the B-factors from the S100B-Ca2+ and S100BCa2+-TRTK12 structures were compared (Figure 6). The B-factors were nearly the same for most residues in the protein with the exception of residues in EF2. Specifically, several residues in EF2 (i.e. S62, D63, G64, D65 and G66) had significantly higher temperature factors when the target peptide was not present (Figure 6). These changes are observed in both models A and B of S100B-Ca2+, thus excluding the possibility of model-specific variations (Fig. 6). One explanation for the change in the B-factor values is that there was one variation in the lattice involving Lys-48 and two residues in EF2 (D65, D67). Specifically, Lys-48 is nearby the backbone carbonyl oxygen of Asp-65 in S100B-Ca2+ (3.26 Å) and may form a very weak hydrogen bond; whereas, in the S100B-Ca2+-TRTK12 structure, the sidechain of Lys-48 is no longer within a hydrogen bond distance of Asp-65, but rather it approaches a carboxylate oxygen atom of Glu-67 (2.79 Å) and may form an ionic interaction instead. Thus, it is possible that this change in a lattice contact between crystallographic symmetry mates may contribute to the variation in B-factor values observed for residues in EF2 when S100B-Ca2+ and S100B-Ca2+-TRTK12 are compared. However, this explanation is unlikely since the binding of a small molecule inhibitor of S100B, pentamidine, also causes the B-factors to lower for residues in EF2 (Fig. 4A and 4B; insets) even though a different lattice interaction is observed. In this structure, one residue at the beginning of the EF2 loop (S62) has two hydrogen bonds across the lattice with a backbone nitrogen atom (G22; 2.65 Å) and carboxylate oxygen atom (E21; 2.66 Å). Another explanation is that a single-body motion involving the loop comprising EF2 (residues S62 to G66) in the Ca2+-bound form of S100B contributes to the lower B-factors and that TRTK-12 binding eliminates such motion. This explanation is supported by the fact that we observe weaker Ca2+ ion binding for S100B in the absence of bound TRTK-12 in solution. Further support for this interpretation is provided by 15N-relaxation rate data published previously in which Glu-62 illustrates fast time-scale motion for S100B-Ca2+ (i.e. via 15N-1H NOE)24. That TRTK-12 binding to Ca2+-S100B can eliminate dynamic motion in EF2 is also provided with NMR data from a mutant of S100B (E72A), which exhibits extensive broadening in EF2 for the Ca2+-bound form of S100B that is eliminated upon binding to TRTK-12. Furthermore, the calcium-affinity in EF2 was significantly reduced for this mutant as was previously established23; however, the highly characteristic downfield glycine proton-15N correlation (G66) in EF2, which is absent in Ca2+-S100B for the E72A mutant of S100B, is rescued and returns clearly when TRTK-12 is bound (Fig. 2S). Such a result is consistent with TRTK-12 binding rescuing the ability of EF2 of the E72A mutant protein to bind Ca2+ tightly such that it is once again interacting with the protein in the slow-exchange regime.
Summary
The S100B-Ca2+-TRTK12 complex was found to have a 5-fold higher affinity for Ca2+ than S100B alone. One possible explanation for increased Ca2+-ion binding affinity explored here is that TRTK-12 binding to S100B-Ca2+ introduced a structural change that increased the number of Ca2+ ligands and/or improved the protein's Ca2+ ion coordination geometry. However, this possibility was ruled out when the structures of S100B-Ca2+-TRTK12 and Ca2+-S100B were compared using X-ray crystallography. Specifically, the calcium liganding residues were found to be the same in both EF-hand domains (S100-EF1, EF2) in the absence or presence of bound TRTK12 (S100-EF1, residues 18–31; EF2, residues 61–72), and the orientation of the Ca2+-coordinating sidechains were nearly identical in both EF-hand domains. However, the B-factors for several residues in the canonical EF-hand calcium binding site (EF2) were significantly lower when TRTK12 was bound to S100B-Ca2+. Furthermore, a similar observation was made when a small molecule inhibitor binds to calcium-S100B. These observations were also consistent with NMR data for which dynamic properties of Ca2+-S100B observed on both fast and slow timescales were eliminated when TRTK-12 or other target peptides bind to S100B-Ca2+23; 24; 32. Thus, a model in which TRTK-12 binding to S100B-Ca2+ eliminates dynamic properties in EF2 and helix 4 is a mechanism that should be considered further as an explanation for the 5-fold increase in calcium-binding affinity when the TRTK-12 peptide target is bound. Lastly, it follows that like TRTK12, any small molecule inhibitor bound to Ca2+-S100B would also have to cause an increase in calcium ion binding affinity to be effective therapeutically inside a cell making these data relevant to future drug-design studies involving S100B.
Material and Methods
Materials
All chemicals and reagents were ACS-grade or higher and typically purchased from Sigma — Aldrich unless otherwise indicated. 15NH4Cl was purchased from Cambridge Isotope Laboratories (Andover, MA). All buffers were passed through Chelex-100 resin to remove trace metals prior to use.
Bacterial expression and purification of the wild-type S100B
Recombinant S100B protein and the E72A mutant were expressed in E. coli (HMS174(DE3) strain) and purified as previously described30. Yields of wild-type and mutant S100B protein were typically 20–30 mg of purified protein per liter of bacterial culture. For NMR experiments, S100B protein was prepared using defined media that included 15N-labeled NH4Cl as the only nitrogen source30.
Peptides
All peptides were synthesized using solid-state peptide synthesis and their purity was determined to be >95% by HPLC and mass spectrometry (Biosynthesis Inc., Lewisville, Texas). The TAMRA-TRTK12-am is an N-terminal 5-carboxytetramethylrhodamine (TAMRA) labeled derivative of the TRTK-12 peptide derived from the actin capping protein, CapZ, residues 265–276 (TAMRA-TRTKIDWNKILS-am) with an amidated C-terminus (-am). The TAMRA-TRTK12-am peptide was suspended in H2O at 450 μM, and the pH adjusted to 7.2 with stocks stored frozen in 50 μL aliquots. The concentration of TAMRA-TRTK12-am was determined at pH 7.2 in H2O using the extinction coefficient for TAMRA, ε547 = 65,000 cm−1M−1. The TRTK-12 peptide was suspended in H2O and then eluted through a G15 column and dialyzed twice in 0.25 mM Tris pH 7.5 and then lyophilized. The powder was suspended in 98% D2O to a final concentration of 6.15 mM TRTK-12 and 4.9 mM Tris-D11 and stored in 50 μL aliquots. The concentration of TRTK-12 was determined at pH 7.5 in 0.5 mM Tris-D11, 98% D2O using the extinction coefficient for TRTK12-am, ε280 = 8,850 cm−1M−1.
Fluorescent polarization competition assay (FPCA)
Fluorescence polarization competition assays (FPCA) were performed in Corning 96-well, flat bottom plates (Corning, NY) in a final volume of 200 μl. The S100B titration into TAMRA-TRTK12-am contained 0 – 40 μM S100B, 50 nM TAMRA-TRTK12-am, 50 mM Tris pH 7.5, 10 mM MnCl2 or CaCl2, and 0.10% Triton X-100. The polarization was read from the top of the well with a PolarStar fluorescent plate reader (BMG Labtech, Durham, NC) using a 544±10 nm excitation and a 590±10 nm emission filter with the temperature maintained at 30°C. Titrations of TRTK-12 into a solution of TAMRA-TRTK12-am bound to Ca2+- or Mn2+-S100B (wild-type or E72A mutant) were performed by monitoring the change in polarization of TAMRA-TRTK12-am with a Varian Cary Eclipse fluorescence spectrophotometer in quartz cuvettes. In these competition binding experiments, the sample contained 5 μM S100B, 50 nM TAMRA-TRTK12, 0–100μM TRTK-12, 50 mM Tris pH 7.2, 15 mM NaCl, 100 mM KCl, 10 mM CaCl2 or MnCl2, 0.10% Triton X-100, and the temperature was maintained at 30°C using a circulating constant-temperature bath. The binding data were fit using a single site binding model with Origin software (OriginLab Corp., Northampton, MA), with one peptide bound per symmetrical S100B subunit. For the fluorescence polarization competition titrations, an equation derived by Nikolovska-Coleska et al. was used to calculate the dissociation constant (KD) using the IC50 values as follows: KD = [I]50/([L]50/TAMARA-TRTK12KD + [P]0/TAMARA-TRTK12KD + 1) where [I]50 is the concentration of the unlabelled TRTK-12 at 50% inhibition, [L]50 is the concentration of the free TAMRA-TRTK12 at 50% inhibition, [P]0 is the concentration of the free protein at 0% inhibition, and TAMRA-TRTK12KD is the dissociation constant for the dissociation of TAMRA-TRTK12-am from the S100B-Ca2+-TAMRA-TRTK12-am complex26.
Metal and TRTK-12 peptide binding to S100B
The concentration of free Mn2+ in a mixture of free and bound Mn2+ was determined by electron paramagnetic resonance27 using a Varian E-4 EPR spectrometer with the temperature maintained at 22°C in a buffer of 50mM Tris at pH 7.2. These data were supplemented by studies of bound Mn2+ under the same conditions by measuring the longitudinal relaxation rates of water protons at 24.3 MHz with a 180°-τ-90° pulse sequence using a Seimco pulsed NMR spectrometer as described previously27; 46. The observed enhancement of the water proton relaxation rate is defined as ε* = (1/T1P*)/(1/T1P) where 1/T1P is the paramagnetic contribution to the longitudinal relaxation rate in the presence (*) and absence (no symbol) of S100B27. The NMR and EPR data were analyzed to determine the stoichiometry (n) of Mn2+ bound, the dissociation constant (KD), and the enhancement factor (εB) of the S100B-Mn2+ complex as previously described21; 27; 47; 48; 49; 50; 51; 52. In addition the binding of Mn2+ (41, 60 and 82 μM) to S100B (65 μM) in the presence of the TRTK-12 (150 μM) peptide (MnK2) was monitored by EPR and by changes in 1/T1P* of water protons and the observed enhancement factor (ε*). A dissociation constant (MnK3) of the TRTK-12 peptide from the S100B-Mn2+-TRTK12 (S100B: 65 μM; TRTK-12: 0–150 μM) complex was analyzed using a noncooperative binding model to find the dissociation constant (MnK3) and enhancement factors (εT) as previously described at two concentrations of Mn2+ (82 and 60 μM)21; 27; 50; 53. The apparent dissociation constants of Ca2+ (0–5mM) from the tight site of S100B (CaKapp) in the absence and presence of TRTK-12 peptide were obtained in competition experiments in which the S100B-Mn2+(±TRTK12) were titrated with Ca2+ and monitoring the displacement of Mn2+ by changes in the enhancement (ε *) of 1/T1P* and independently by the appearance of free Mn2+ in the EPR spectrum as previously described 21. There was no detectable binding for EF1 (pseudo EF hand) when TRTK-12 was present as seen previously for the p53 peptide21; therefore, the subsequent competition experiments with Ca2+ only report on the binding to the tight site (EF2). In addition, any binding of calcium to EF1 that may occur at the highest calcium concentrations, in such competition experiments, do not significantly affect the total calcium available to bind EF2 (< 1%). Thus, any effect of EF1 sequestering small amounts of calcium, under the conditions used in these experiments, have no effect on the calculations of K2 values for EF2, within the error limits reported (Table 1). From these data, the actual dissociation constants of Ca2+ from the S100B-Mn2+(±TRTK12) were calculated using the competition equation CaKD=CaKapp/(1+([Mn2+]/Mn-CaKD)). All calcium stocks were prepared from a calibrated 1.00 M CaCl2 standard stock that was purchased from Fluka Analytical and the MnCl2 concentration was determined by comparing the 1/T1P of stock solutions to that of a Mn2+ sample of known concentration as previously described27.
Protein crystallization
Bovine S100B protein was dialyzed into buffer (0.1 mM TES, pH 7.2, and 0.05 mM DTT), lyophilized, and dissolved in ddH2O to 80–100 mg/mL (~ 8–10 mM subunit concentration) and stored frozen. Diffraction quality crystals for the S100B-Ca2+-TRTK12 complex were obtained by sitting drop vapor diffusion at 22 °C by mixing 2 μL of S100B protein (40 mg/mL S100B, 7.5 mM CaCl2, 3.8 mM TRTK-12, 20 mM cacodylate buffer pH 7.2) with 2 μL of reservoir solution (7.5 mM CaCl2, 0.1 M cacodylate buffer pH 6.8, and 26% PEG3350) and equilibrating for 2–3 days. After crystals formed, they were cryoprotected in a harvest solution (7.5 mM CaCl2, 3.8 mM TRTK-12, 0.07 M cacodylate buffer pH 6.8, 28% PEG3350, and 5% glycerol) for 30–60 seconds and then flash-cooled in liquid nitrogen. The space group and unit cell parameters are given in Table 2. The above crystal forms had one S100B-Ca2+-TRTK12 complex subunit in the asymmetric unit.
Diffraction quality crystals for the S100B-Ca2+ were obtained in a similar manner as S100B-Ca2+-TRTK12 with 2 μL of S100B protein sample buffer (40 mg/mL S100B, 15 mM CaCl2, 20 mM cacodylate buffer pH 6.5) and 2 μL of reservoir solution (7.5 mM CaCl2, 0.1 M cacodylate buffer pH 6.3, and 25% PEG3350) and were cryoprotected (15 mM CaCl2, 0.1 M cacodylate buffer pH 6.3, 28% PEG3350, and 5% glycerol). The space group was C2 (Table 2) which was different and a lower symmetry than the previously published S100B-Ca2+ (1MHO)12 and S100B-Ca2+-TRTK12 that had a spacegroup of C2221. The above crystal form of S100B-Ca2+ had two monomer subunits, which are labeled model A and model B in the asymmetric unit.
X-ray data collection, model building, and refinement
X-ray data for S100BCa2+-TRTK12 were collected at 100 K using an in-house X-ray generator (MSC micromax 7; Rigaku Texas, USA) and a Raxis4++ image plate detector (Rigaku Texas, USA). X-ray data for S100B-Ca2+ were collected remotely at the SSRL 12–2 beamline (Stanford Synchrotron Radiation Laboratory, Menlo Park, CA). The reflection intensities were integrated and scaled with the HKL2000 suite of computer programs54. The crystal of S100B-Ca2+ and S100B-Ca2+-TRTK12 diffracted to 1.50 Å and 2.01 Å resolutions, respectively. Both structures were solved by molecular replacement using the structure of Ca2+-bound S100B with calcium ions removed (PDB file: 1MHO;12) as a search model and the computer program PHASER from the CCP4 program suite55; 56. Model building and refinement of the S100B structure were completed using COOT and REFMAC557; 58. The locations of the TRTK-12 molecule and several water molecules were determined by visual inspection electron density maps calculated with the 2mFo-DFc and mFo-DFc coefficients with COOT58. It was evident from visual inspection of the 2mFo-DFc and mFo-DFc electron density maps that the only way to accurately model the bound TRTK-12 was to remove residues Thr-1 and Arg-2 from the N-terminal side and Ser-12 from the C-terminal side from the model. The C-terminal region of TRTK-12 is found on the crystallographic two-fold axis affecting Thr-1 and Arg-2, and there was no convincing density for these residues. The residue Thr-3 was found at the crystallographic two-fold axis with convincing density for this residue, where the atoms Cα and Cβ of Thr-3 were set to an occupancy value of 0.5 by REFMAC5. The stereochemistry was checked with the programs WHATCHECK and PROCHEK59; 60. The quaternary structure and accessible surface areas were analyzed using the PISA server (http://www.ebi.ac.uk/msd-srv/prot_int/cgi-bin/piserver). Figures were generated with the program PyMol (http://www.pymol.org).
NMR Spectroscopy
Purified 15N-labeled S100B was dialyzed against 0.1 mM TES pH 7.2, lyophilized, and hydrated in a small aliquot of ddH2O and stored at −80 or −20 °C. The apo and Ca2+-loaded E72A S100B mutant NMR sample was prepared in a manner similar to that previously described23 and contained 0.25 mM E72A mutant S100B subunit concentration, 0.625 mM TRTK-12, 0.34 mM NaN3, 15 mM NaCl, 15 mM CaCl2, 10% D2O, 10 mM TES buffer, and adjusted to pH 7.2 with HCl. The Apo-S100B sample contained 1 mM EDTA, and the resonance assignments determined previously for the E72A mutant of S100B in the apo and Ca2+-bound states were confirmed here with standard 3D 15N-edited NOESY and 15N-edited HMQC-NOESY-HSQC23. Likewise, the observable backbone 1H and 15N assignments for the E72A mutant of S100B-Ca2+ bound to TRTK-12 were assigned in a straightforward manner in 2D HSQC titrations and confirmed using 3D 15N-edited NOESY and 15N-edited HMQC-NOESY-HSQC. Heteronuclear single quantum coherence (HSQC) NMR data, 3D 15N-edited NOESY, and 15N-edited HMQC-NOESY-HSQC data were collected at 37 °C with an Avance 800 US2 (800.27 MHz for protons) NMR spectrometer both equipped with pulsed-field gradients, four frequency channels, and triple resonance, z-axis gradient cryogenic probes63. Data were processed with NMRPipe and analyzed with nmrDraw and NMRViewJ64; 65. Proton chemical shifts were reported with respect to the H2O or HDO signal taken as 4.658 ppm relative to external TSP (0.0 ppm). The 15N chemical shifts were indirectly referenced as previously described using the following ratio of the zero-point frequency: 0.10132905 for 15N to 1H66; 67; 68.
Supplementary Material
Supplementary Figure 1. Plots of electron paramagnetic resonance (EPR) measurements of free Mn2+ in the absence and presence of S100B and the TRTK-12 peptide. For the S100B-Mn2+-TRTK-12 complex, the solution contained S100B (65 μM), TRTK-12 (1.5 mM), MnCl2 (82 μM) in 50 mM Tris, pH 7.2. For the S100B-Mn2+ complex, the solution contained S100B (65 μM), MnCl2 (82 μM) in 50 mM Tris, pH 7.2. For the measurement of TRTK-12 and Mn2+ (control), the solution contained TRTK-12 (1.5 mM), MnCl2 (82 μM) in 50 mM Tris, pH 7.2. Finally, the Mn2+ alone solution (control 2), the solution contained MnCl2 (82 μM) in 50 mM Tris at pH 7.2.
Supplementary Figure 2. Binding of TRTK-12 to the E72A mutant of S100B. Displacement of the TAMRA-TRTK12 peptide from the E72A S100B-Ca2+-TAMRA-TRTK12 complex by unlabeled TRTK12 as monitored by fluorescence polarization. The solution contained 1.5 μM E72A S100B, 50 nM TAMRA-TRTK12, and 10 mM CaCl2 in 50mM Tris-HCl pH 7.5. The curve represents a Kapp value of 109.6 ± 10.3 μM and TAMRA-TRTK12 was found to bind to the E72A mutant with a KD of 16.8 ± 3.1 μM; these titrations were repeated in triplicate and used to determine the dissociation of TRTK12 from the E72A-Ca2+-TRTK12 complex (KD= 3.2 ± 1.5 μM) as described in Methods. (Inset) NMR heteronuclear single quantum coherence (HSQC) spectra of wild-type S100B (black; control) and E72A mutant of S100B (red) in the presence of calcium and TRTK-12 (inset). In the absence of TRTK-12, the E72A mutant of S100B showed no downfield HSQC correlation for glycine-66 (G66) indicating that the Ca2+ binding properties of this mutant were significantly weakened in the absence of TRTK-12. For wild-type S100B (Black), the solution contained wild-type S100B (100 μM), Ca2+ (20 mM) and TRTK-12 (250 μM). For the E72A mutant of S100B, the solution contained E72A S100B (250 μM), Ca2+ (20 mM), and TRTK-12 peptide (625 μM).
Acknowledgment
We thank the staff of the 12–2 beamline at the Stanford Synchrotron Radiation Lightsource for their assistance in collecting X-ray diffraction data. We are also grateful to Dr. Albert S. Mildvan for his generous assistance with the electron paramagnetic resonance (EPR) and proton relaxation rate measurements (PRR) collected at Johns Hopkins University School of Medicine.
This work was supported with grants from the National Institutes of Health GM58888 (DJW), CA107331 (DJW), and the American Cancer Society CDD107745 (DJW).
Abbreviations
- EPR
electron paramagnetic resonance
- NMR
Nuclear Magnetic Resonance
- DTT
dithiothreitol
- TPPI
time-proportional phase incrimination
- TSP
3-(trimethylsilyl)-propionic acid-D4 sodium salt
- PEG
polyethylene glycol
- TAMRA
Carboxytetramethylrhodamine
- HSQC
heteronuclear single-quantum coherence
- EDTA
ethylenediaminetetraacetic acid
- NOE
Nuclear Overhauser Effect
- TES
(2-[2-hydroxyl-1,1-bis[hydroxymethyl]ethyl)-amino]ethanesulfonic acid)
- Tris-HCl
tris(hydroxymethyl)-aminomethane hydrochloride
- ddH2O
deionized and doubly distilled H2O
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore B. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Comm. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 2.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–51. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 3.Heizmann CW. The multifunctional S100 protein family. Methods Mol Biol. 2002;172:69–80. doi: 10.1385/1-59259-183-3:069. [DOI] [PubMed] [Google Scholar]
- 4.Wilder PT, Lin J, Bair CL, Charpentier TH, Yang D, Liriano M, Varney KM, Lee A, Oppenheim AB, Adhya S, Carrier F, Weber DJ. Recognition of the tumor suppressor protein p53 and other protein targets by the calcium-binding protein S100B. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbamcr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–14. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drohat AC, Amburgey JC, Abildgaard F, Starich MR, Baldisseri D, Weber DJ. Solution structure of rat apo-S100B as determined by NMR spectroscopy. Biochemistry. 1996;35:11577–11588. doi: 10.1021/bi9612226. [DOI] [PubMed] [Google Scholar]
- 7.Drohat AC, Baldisseri DM, Rustandi RR, Weber DJ. Solution structure of calcium-bound rat S100B(betabeta) as determined by nuclear magnetic resonance spectroscopy. Biochemistry. 1998;37:2729–40. doi: 10.1021/bi972635p. [DOI] [PubMed] [Google Scholar]
- 8.Drohat AC, Tjandra N, Baldisseri DM, Weber DJ. The use of dipolar couplings for determining the solution structure of rat apo-S100B. Protein Science. 1999;8:800–9. doi: 10.1110/ps.8.4.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inman KG, Yang R, Rustandi RR, Miller KE, Baldisseri DM, Weber DJ. Solution NMR structure of S100B bound to the high-affinity target peptide TRTK-12. Journal of Molecular Biology. 2002;324:1003–14. doi: 10.1016/s0022-2836(02)01152-x. [DOI] [PubMed] [Google Scholar]
- 10.Rustandi RR, Baldisseri DM, Weber DJ. Structure of the negative regulatory domain of p53 bound to S100B. Nat Struct Biol. 2000;7:570–4. doi: 10.1038/76797. [DOI] [PubMed] [Google Scholar]
- 11.Kilby PM, Van Eldik LJ, Roberts GC. The solution structure of the bovine S100B protein dimer in the calcium-free state. Structure. 1996;4:1041–52. doi: 10.1016/s0969-2126(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura H, Shiba T, Inoue T, Harada S, Kai Y. A novel mode of target recognition suggested by the 2.0 A structure of holo S100B from bovine brain. Structure. 1998;6:233–41. doi: 10.1016/s0969-2126(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 13.Smith SP, Shaw GS. A novel calcium-sensitive switch revealed by the structure of human S100B in the calcium-bound form. Structure. 1998;6:211–222. doi: 10.1016/s0969-2126(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 14.Drohat AC, Nenortas E, Beckett D, Weber DJ. Oligomerization state of S100B at nanomolar concentration determined by large-zone analytical gel filtration chromatography. Protein Sci. 1997;6:1577–82. doi: 10.1002/pro.5560060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson PL, Shaw GS. Role of the N-terminal helix I for dimerization and stability of the calcium-binding protein S100B. Biochemistry. 2002;41:3637–46. doi: 10.1021/bi0118052. [DOI] [PubMed] [Google Scholar]
- 16.Amburgey JC, Abildgaard F, Starich MR, Shah S, Hilt DC, Weber DJ. 1H, 13C and 15N NMR assignments and solution secondary structure of rat Apo-S100 beta. J Biomol NMR. 1995;6:171–9. doi: 10.1007/BF00211781. [DOI] [PubMed] [Google Scholar]
- 17.Strynadka NCJ, James MNG. Crystal structures of the helix-loop-helix calcium-binding proteins. Ann. Rev. Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 18.Kretsinger RH, Rudnick SE, Sneden DA, Schatz VB. Calmodulin, S-100, and crayfish sarcoplasmic calcium-binding protein crystals suitable for X-ray diffraction studies. Journal of Biological Chemistry. 1980;255:8154–6. [PubMed] [Google Scholar]
- 19.Baudier J, Gerard D. Ions binding to S100 proteins. II. Conformational studies and calcium-induced conformational changes in S100 alpha alpha protein: the effect of acidic pH and calcium incubation on subunit exchange in S100a (alpha beta) protein. J Biol Chem. 1986;261:8204–12. [PubMed] [Google Scholar]
- 20.Weber DJ, Rustandi RR, Carrier F, Zimmer DB. Interaction of dimeric S100B(ββ) with the tumor suppressor protein: A model for Ca-dependent S100-target protein interactions. In: Pochet R, editor. The molecular basis of calcium action in biology and medicine. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. [Google Scholar]
- 21.Rustandi RR, Drohat AC, Baldisseri DM, Wilder PT, Weber DJ. The Ca2+-dependent interaction of S100B with a peptide derived from p53. Biochemistry. 1998;37:1951–60. doi: 10.1021/bi972701n. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz J, Rustandi RR, Varney KM, Wilder PT, Udan R, Wu SL, Horrocks WD, Weber DJ. Calcium-Binding Properties of Wild-Type and EF-Hand Mutants of S100B in the Presence and Absence of a Peptide Derived from the C-Terminal Negative Regulatory Domain of p53. Biochemistry. 2005;44:7305–7314. doi: 10.1021/bi050321t. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz J, Rustandi RR, Varney KM, Wilder PT, Udan R, Wu SL, Horrocks WD, Weber DJ. Calcium-binding properties of wild-type and EF-hand mutants of S100B in the presence and absence of a peptide derived from the C-terminal negative regulatory domain of p53. Biochemistry. 2005;44:7305–14. doi: 10.1021/bi050321t. [DOI] [PubMed] [Google Scholar]
- 24.Wright NT, Inman KG, Levine JA, Cannon BR, Varney KM, Weber DJ. Refinement of the solution structure and dynamic properties of Ca(2+)-bound rat S100B. J Biomol NMR. 2008;42:279–86. doi: 10.1007/s10858-008-9282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mildvan AS, Granot J, Smith GM, Liebman M. Adv. Inorg. Biochem. 1979;2:211–236. [Google Scholar]
- 26.Nikolovska-Coleska Z, Wang R, Fang X, Pan H, Tomita Y, Li P, Roller PP, Krajewski K, Saito NG, Stuckey JA, Wang S. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal Biochem. 2004;332:261–73. doi: 10.1016/j.ab.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 27.Mildvan AS, Engle JL. Nuclear relaxation measurements of water protons and other ligands. Methods Enzymol. 1972;26(PtC):654–82. doi: 10.1016/s0076-6879(72)26031-1. [DOI] [PubMed] [Google Scholar]
- 28.Mildvan AS, Engle JL. Nuclear relaxation measurements of water protons and other ligands. Methods Enzymol. 1972;49D:322–359. doi: 10.1016/s0076-6879(72)26031-1. [DOI] [PubMed] [Google Scholar]
- 29.Rustandi RR, Drohat AC, Baldisseri DM, Wilder PT, Weber DJ. The Ca(2+)-dependent interaction of S100B(beta beta) with a peptide derived from p53. Biochemistry. 1998;37:1951–60. doi: 10.1021/bi972701n. [DOI] [PubMed] [Google Scholar]
- 30.Drohat AC, Baldisseri DM, Rustandi RR, Weber DJ. Solution structure of calcium-bound rat S100B as determined by nuclear magnetic resonance spectroscopy. Biochemistry. 1998;37:2729–2740. doi: 10.1021/bi972635p. [DOI] [PubMed] [Google Scholar]
- 31.McClintock KA, Shaw GS. A novel S100 target conformation is revealed by the solution structure of the Ca2+-S100B-TRTK-12 complex. J Biol Chem. 2003;278:6251–7. doi: 10.1074/jbc.M210622200. [DOI] [PubMed] [Google Scholar]
- 32.Rustandi RR, Baldisseri DM, Drohat AC, Weber DJ. Structural changes in the C-terminus of Ca2+-bound rat S100B upon binding to a peptide derived from the C-terminal regulatory domain of p53. Protein Science. 1999;8:1743–1751. doi: 10.1110/ps.8.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inman KG, Yang R, Rustandi RR, Miller KE, Baldisseri DM, Weber DJ. Solution NMR structure of S100B bound to the high-affinity target peptide TRTK-12. J Mol Biol. 2002;324:1003–14. doi: 10.1016/s0022-2836(02)01152-x. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharya S, Large E, Heizmann CW, Hemmings B, Chazin WJ. Structure of the Ca2+/S100B/NDR kinase peptide complex: insights into S100 target specificity and activation of the kinase. Biochemistry. 2003;42:14416–26. doi: 10.1021/bi035089a. [DOI] [PubMed] [Google Scholar]
- 35.Wilder PT, Lin J, Bair CL, Charpentier TH, Yang D, Liriano M, Varney KM, Lee A, Oppenheim AB, Adhya S, Carrier F, Weber DJ. Recognition of the tumor suppressor protein p53 and other protein targets by the calcium-binding protein S100B. Biochim Biophys Acta. 2006;1763:1284–97. doi: 10.1016/j.bbamcr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 36.McClintock KA, Van Eldik LJ, Shaw GS. The C-terminus and linker region of S100B exert dual control on protein-protein interactions with TRTK-12. Biochemistry. 2002;41:5421–8. doi: 10.1021/bi011732m. [DOI] [PubMed] [Google Scholar]
- 37.Otterbein LR, Kordowska J, Witte-Hoffmann C, Wang CL, Dominguez R. Crystal structures of S100A6 in the Ca(2+)-free and Ca(2+)-bound states: the calcium sensor mechanism of S100 proteins revealed at atomic resolution. Structure. 2002;10:557–67. doi: 10.1016/s0969-2126(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhuri D, Horrocks WD, Jr., Amburgey JC, Weber DJ. Characterization of lanthanide ion binding to the EF-hand protein S100 beta by luminescence spectroscopy. Biochemistry. 1997;36:9674–80. doi: 10.1021/bi9704358. [DOI] [PubMed] [Google Scholar]
- 39.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–68. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 40.Heizmann CW, Cox JA. New perspectives on S100 proteins: a multi-functional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. Biometals. 1998;11:383–97. doi: 10.1023/a:1009212521172. [DOI] [PubMed] [Google Scholar]
- 41.Ivanenkov VV, Jamieson GA, Jr., Gruenstein E, Dimlich RV. Characterization of S-100b binding epitopes. Identification of a novel target, the actin capping protein, CapZ. J Biol Chem. 1995;270:14651–8. doi: 10.1074/jbc.270.24.14651. [DOI] [PubMed] [Google Scholar]
- 42.Delphin C, Ronjat M, Deloulme JC, Garin G, Debussche L, Higashimoto Y, Sakaguchi K, Baudier J. Calcium-dependent interaction of S100B with the C-terminal domain of the tumor suppressor p53. J Biol Chem. 1999;274:10539–44. doi: 10.1074/jbc.274.15.10539. [DOI] [PubMed] [Google Scholar]
- 43.Wright NT, Cannon BR, Wilder PT, Morgan MT, Varney KM, Zimmer DB, Weber DJ. Solution structure of S100A1 bound to the CapZ peptide (TRTK12) J Mol Biol. 2009;386:1265–77. doi: 10.1016/j.jmb.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J, Blake M, Tang C, Zimmer D, Rustandi RR, Weber DJ, Carrier F. Inhibition of p53 transcriptional activity by the S100B calcium-binding protein. J Biol Chem. 2001;276:35037–41. doi: 10.1074/jbc.M104379200. [DOI] [PubMed] [Google Scholar]
- 45.Lin J, Yang Q, Yan Z, Markowitz J, Wilder PT, Carrier F, Weber DJ. Inhibiting S100B Restores p53 Levels in Primary Malignant Melanoma Cancer Cells. J Biol Chem. 2004;279:34071–7. doi: 10.1074/jbc.M405419200. [DOI] [PubMed] [Google Scholar]
- 46.Carr HY, Purcell EM. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954;94:630–638. [Google Scholar]
- 47.Mildvan AS, Cohn M. Kinetic and magnetic resonance studies of the pyruvate kinase reaction. II. Complexes of enzyme, metal, and substrates. J Biol Chem. 1966;241:1178–93. [PubMed] [Google Scholar]
- 48.Mildvan AS, Cohn M. Magnetic Resonance Studies of the Interaction of the Manganous Ion with Bovine Serum Albumin. Biochemistry. 1963;2:910–9. doi: 10.1021/bi00905a003. [DOI] [PubMed] [Google Scholar]
- 49.Serpersu EH, Shortle D, Mildvan AS. Kinetic and magnetic resonance studies of effects of genetic substitution of a Ca2+-liganding amino acid in staphylococcal nuclease. Biochemistry. 1986;25:68–77. doi: 10.1021/bi00349a011. [DOI] [PubMed] [Google Scholar]
- 50.Serpersu EH, Shortle D, Mildvan AS. Kinetic and magnetic resonance studies of active-site mutants of staphylococcal nuclease: factors contributing to catalysis. Biochemistry. 1987;26:1289–300. doi: 10.1021/bi00379a014. [DOI] [PubMed] [Google Scholar]
- 51.Weber DJ, Meeker AK, Mildvan AS. Interactions of the acid and base catalysts on staphylococcal nuclease as studied in a double mutant. Biochemistry. 1991;30:6103–14. doi: 10.1021/bi00239a004. [DOI] [PubMed] [Google Scholar]
- 52.Weber DJ, Serpersu EH, Shortle D, Mildvan AS. Diverse interactions between the individual mutations in a double mutant at the active site of staphylococcal nuclease. Biochemistry. 1990;29:8632–42. doi: 10.1021/bi00489a020. [DOI] [PubMed] [Google Scholar]
- 53.Reed GH, Cohn M, O'Sullivan WJ. Analysis of equilibrium data from proton magnetic relaxation rates of water for manganese-nucleotide-kinase ternary complexes. J Biol Chem. 1970;245:6547–52. [PubMed] [Google Scholar]
- 54.Otwinowski Z, Minor W. Processing of X-Ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 55.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vagin AA, Isupov MN. Spherically averaged phased translation function and its application to the search for molecules and fragments in electron-density maps. Acta Crystallogr D Biol Crystallogr. 2001;57:1451–6. doi: 10.1107/s0907444901012409. [DOI] [PubMed] [Google Scholar]
- 57.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–55. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 58.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 59.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. Journal of Applied Crystallography. 1993;26:283–91. [Google Scholar]
- 60.Laskowski RA, MacArthur MW, Thornton JM. Validation of protein models derived from experiment. Curr Opin Struct Biol. 1998;8:631–9. doi: 10.1016/s0959-440x(98)80156-5. [DOI] [PubMed] [Google Scholar]
- 61.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–42. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang H, Guranovic V, Dutta S, Feng Z, Berman HM, Westbrook JD. Automated and accurate deposition of structures solved by X-ray diffraction to the Protein Data Bank. Acta Crystallogr D Biol Crystallogr. 2004;60:1833–9. doi: 10.1107/S0907444904019419. [DOI] [PubMed] [Google Scholar]
- 63.Mori S, Abeygunawardana C, Johnson MO, van Zijl PC. Improved sensitivity of HSQC spectra of exchanging protons at short interscan delays using a new fast HSQC (FHSQC) detection scheme that avoids water saturation. J Magn Reson B. 1995;108:94–8. doi: 10.1006/jmrb.1995.1109. [DOI] [PubMed] [Google Scholar]
- 64.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–93. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 65.Johnson BA. Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol Biol. 2004;278:313–52. doi: 10.1385/1-59259-809-9:313. [DOI] [PubMed] [Google Scholar]
- 66.Edison AS, Abildgaard F, Westler WM, Mooberry ES, Markley JL. Practical introduction to theory and implementation of multinuclear, multidimensional nuclear magnetic resonance experiments. Methods Enzymol. 1994;239:3–79. doi: 10.1016/s0076-6879(94)39003-7. [DOI] [PubMed] [Google Scholar]
- 67.Live DH, Davis DG, Agosta WC, Cowburn D. Longe range hydrogen bond mediated effects in peptides: 15N NMR study of gramicidin S in water and organic solvents. J. Am. Chem. Soc. 1984;106:1939–1941. [Google Scholar]
- 68.Spera S, Bax A. Empirical correlation between protein backbone conformation and Cα and Cβ/13-C nuclear magnetic resonance chemical shifts. Journal of American Chemical Society. 1991;113:5490–5492. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Plots of electron paramagnetic resonance (EPR) measurements of free Mn2+ in the absence and presence of S100B and the TRTK-12 peptide. For the S100B-Mn2+-TRTK-12 complex, the solution contained S100B (65 μM), TRTK-12 (1.5 mM), MnCl2 (82 μM) in 50 mM Tris, pH 7.2. For the S100B-Mn2+ complex, the solution contained S100B (65 μM), MnCl2 (82 μM) in 50 mM Tris, pH 7.2. For the measurement of TRTK-12 and Mn2+ (control), the solution contained TRTK-12 (1.5 mM), MnCl2 (82 μM) in 50 mM Tris, pH 7.2. Finally, the Mn2+ alone solution (control 2), the solution contained MnCl2 (82 μM) in 50 mM Tris at pH 7.2.
Supplementary Figure 2. Binding of TRTK-12 to the E72A mutant of S100B. Displacement of the TAMRA-TRTK12 peptide from the E72A S100B-Ca2+-TAMRA-TRTK12 complex by unlabeled TRTK12 as monitored by fluorescence polarization. The solution contained 1.5 μM E72A S100B, 50 nM TAMRA-TRTK12, and 10 mM CaCl2 in 50mM Tris-HCl pH 7.5. The curve represents a Kapp value of 109.6 ± 10.3 μM and TAMRA-TRTK12 was found to bind to the E72A mutant with a KD of 16.8 ± 3.1 μM; these titrations were repeated in triplicate and used to determine the dissociation of TRTK12 from the E72A-Ca2+-TRTK12 complex (KD= 3.2 ± 1.5 μM) as described in Methods. (Inset) NMR heteronuclear single quantum coherence (HSQC) spectra of wild-type S100B (black; control) and E72A mutant of S100B (red) in the presence of calcium and TRTK-12 (inset). In the absence of TRTK-12, the E72A mutant of S100B showed no downfield HSQC correlation for glycine-66 (G66) indicating that the Ca2+ binding properties of this mutant were significantly weakened in the absence of TRTK-12. For wild-type S100B (Black), the solution contained wild-type S100B (100 μM), Ca2+ (20 mM) and TRTK-12 (250 μM). For the E72A mutant of S100B, the solution contained E72A S100B (250 μM), Ca2+ (20 mM), and TRTK-12 peptide (625 μM).