Abstract
Effective clearance of inhaled pathogens is the primary innate defense mechanism in the lung, and requires the maintenance of a proper airway surface liquid (ASL) volume to facilitate ciliary beat and optimize mucociliary clearance. Na+ absorption via the epithelial sodium channel (ENaC) is tightly regulated and, together with chloride movement, provides the optimal osmotic gradients to absorb excessive fluid in the airway lumen while preventing excessive ASL dehydration, which would compromise mucus clearance from the lung. To absorb excessive fluid from the luminal surface, a local mechanism of ENaC activation allows for an increase in Na+ absorption at times when the ASL volume is expanded. To help define these regulatory mechanisms, we examined the effects of ASL volume expansion on ENaC activity in primary human bronchial epithelial (HBE) cell cultures. We found that ENaC activity increases dramatically after rapid dilution of endogenous ASL. Approximately 35% of the increase in Na+ absorption was attributable to activation of ENaC by proteases. The remainder of the increase in Na+ current was prevented when membrane trafficking was disrupted with brefeldin A, nocodazole, or myosin light chain kinase inhibitors, demonstrating that trafficking is involved with ENaC regulation in the airway. These findings demonstrate that Na+ absorption in the airway is acutely modulated by the coordinated trafficking of channels to the luminal surface and by the proteolytic activation of ENaC in response to ASL volume expansion.
Keywords: ENaC, HBE, airway epithelium, airway surface liquid
The luminal surfaces of the conducting airways are lined with a thin layer of fluid known as the airway surface liquid (ASL), which facilitates mucus clearance from the lung. The height of the ASL is determined by the net osmotic gradient established by Na+ absorption and Cl− secretion through apically located ion channels (1–4). The epithelial sodium channel (ENaC), in conjunction with basolaterally located Na+/K+ ATPase, is thought to be the predominant means of Na+ absorption across the airway epithelium. ENaC activity in renal and colonic epithelia is dictated by both local and systemic stimuli, whereas ENaC activity in airway epithelium appears to be regulated primarily by local factors in the luminal environment (5). This localized regulation facilitates ENaC autoregulation to maintain an optimal ASL volume. Evidence to date indicates that proteolytic activation (6–8), flow activation (9, 10), and cyclic nucleotides/purino-receptor regulation (11–13) appear to be the predominant mechanisms that regulate airway ENaC under physiologic conditions, rather than responses to hormonal stimuli.
Previous work from our group and others indicates that a balance between the protease activity of membrane-tethered, channel-activating proteases (CAPs) and soluble protease inhibitors in the ASL modulates ENaC activity and therefore Na+ absorption across human bronchial epithelial (HBE) cells (6, 7). When the ASL volume is low, during steady-state and nonpathologic conditions, the concentration of soluble protease inhibitor is sufficient to minimize the constitutive activation of ENaC by CAPs. Conversely, when the ASL volume is expanded, the soluble protease inhibitors are diluted, allowing for CAP-mediated activation of ENaC. In previous work, Na+ absorption in HBE cells under ASL volume expansion conditions was recognized to be greater than that found in control cultures with basal ASL volumes that were exposed to exogenous channel activating proteases (6). This suggested that alternative mechanisms, in addition to proteolytic activation, are present that significantly augment Na+ absorption in the airway in response to ASL expansion.
Na+ absorption via the ENaC is enhanced through either an increase in the open probability (PO) of the channel or through an increase in channel number (n) (14). Although proteolytic processing, flow activation, and cyclic nucleotides/purino-receptor regulation are some mechanisms that alter the PO of the channel, the contribution of ENaC trafficking on the regulation of Na+ absorption in the airway epithelium is unknown. Therefore, this study sought to determine the relative contributions of proteolytic processing and trafficking of ENaC in response to increases in the ASL volume in primary HBE cells. To measure acute changes in ENaC activity in response to ASL volume expansion, we continuously measured the short circuit current (ISC) of HBE cultures after the transition from an air–liquid interface to a liquid–liquid environment in modified Ussing chambers. The rate of increase in ENaC activity after apical submersion was examined in HBE cells cultured in the presence and absence of a variety of steroids, protease inhibitors, and proteases, to determine the degree to which proteolytic regulation modulates channel activity. Because only a portion of the increase in the amiloride-sensitive ISC (INa) could be attributed to protease-mediated activation, we next determined the effects of various blockers of membrane trafficking on the activation of ENaC. In a final series of experiments, we investigated whether manipulation of adenosine receptor signaling, cyclic adenosine monophosphate (cAMP), protein kinase A/C (PKA/PKC), intracellular Ca+, new protein synthesis, or luminal osmolarity altered the acute stimulation of INa after the washout of endogenous ASL. Our findings demonstrate that ENaC regulation in the airway occurs via multiple mechanisms, and suggest that after ASL expansion, a reserve pool of channels is trafficked to the luminal surface where channels can be activated further via proteolytic processing.
MATERIALS AND METHODS
Primary Human Airway Epithelial Cell Culture
HBE cells were cultured from excess pathologic tissue after lung transplantation and organ donation, under a protocol approved by the University of Pittsburgh Investigational Review Board, as previously described (15). Bronchi from the second to sixth generations were dissected, thoroughly rinsed, and incubated overnight at 4°C in Eagle's minimum essential media (EMEM). The bronchi were then digested overnight at 4°C in MEM containing 0.1% protease XIV and 0.01% DNase. The epithelial cells were removed from the underlying stroma by blunt dissection, isolated by centrifugation, and washed in MEM containing 5% FBS. After centrifugation, cells were resuspended in bronchial epithelial growth medium (BEGM; Lonza, Basel, Switzerland) and plated onto Type VI human placental collagen-treated tissue culture flasks. On reaching 80–90% confluence, the passage 0 cells were trypsinized (0.1%), resuspended in BEGM, and seeded onto human placental collagen-coated Costar Transwell filters (0.33 cm2 0.4 μm pore, catalogue number 3470, Corning, Lowell, MA) at a density of approximately 2 × 105/cm2. Upon reaching confluence, the apical media was aspirated, and HBE cultures were maintained at an air–liquid interface (ALI) and fed basolaterally with differentiation media twice weekly. The differentiation media (BEGM/Ultroser G; Pall Corporation, Crescent Chemical Company, Islandia, NY) contained 5 μg/ml insulin, 10 μg/ml transferrin, 0.07 μg/ml hydrocortisone, 0.6 μg/ml epinephrine, 0.8% vol/vol bovine hypothalamus extract, 0.5 mg/mL BSA, 0.5 μM ethanolamine, 15 ng/ml retinoic acid, 0.5 ng/ml human epidermal growth factor, 10 nM triiodothyronine, 0.5 μM phosphoethanolamine, and 0.5% vol/vol Ultroser G (USG) in Dulbecco's MEM (DMEM)/F12. Cells were studied after 3 to 6 weeks of culture, and considered differentiated when a mucociliary phenotype was apparent on phase contrast microscopy.
ISC Recordings
Differentiated HBE cultures were mounted in Ussing chambers (P2300; Physiological Instruments, San Diego, CA), with custom sliders modified to fit the Transwell inserts, and cultures were continuously short-circuited with an automatic voltage clamp (VCC MC8, Physiological Instruments). The Ringer's solution bath consisted of 120 mM NaCl, 25 mM NaHCO3, 3.3 mM KH2PO4, 0.8 mM K2HPO4, 1.2 mM MgCl2, 1.2 mM CaCl2, and 10 mM glucose. Chambers were constantly gassed with a mixture of 95% O2/5% CO2 at 37°C, which maintained the pH at 7.4 and established a circulating perfusion bath within the Ussing chamber. The apical and basolateral chambers each contained 3 ml of Ringer's solution. Simultaneous transepithelial resistance was recorded by applying a 10-mV pulse per second via an automated pulse generator. Acquire and Analyze 2.3 (Physiological Instruments) was used to control the voltage clamp and analyze ISC data. A typical ISC recording included a 30-minute equilibration period, followed by stimulation with 300 nM porcine pancreatic elastase (catalogue number EC134; EPC, Owensville, MO) or 1 μM trypsin. The INa was determined by the addition of 10 μM amiloride to the apical cell chamber at the end of each recording. Additional substances included 50 nM dexamethasone, 50 nM aldosterone, 10 μM forskolin, 100 μM bumetanide, 10 μM aprotinin, 50 μM furin convertase inhibitor (FCI, dec-RVKR-cmk; Axxora, San Diego, CA), A2a-receptor antagonist (4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol; Tocris Bioscience, Ellisville, MO), 50 μM H-7, 10 μM bis(2-aminophenoxy)ethane tetraacetic acid–cetoxymethyl ester (BAPTA-AM) (Invitrogen, Carlsbad, CA), 200 μg/mL cycloheximide (CHX), 5 μg/mL brefeldin A (BFA), 10 μM nocodazole, 100 μM ML-7, 100 μM ML-9, 1 μM wortmannin (Calbiochem, Gibbstown, NJ), nystatin, 200 μM ouabain, and 50 μg/ml vinblastine. Unless otherwise noted, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Determination of ISC Kinetics and ENaC Functional Half-Life
To determine the kinetics of ISC changes, the ISC values for each individual trace over the indicated time interval was fit to the exponential equation y = y0 + a*(1 − e−t/τ), where y = ISC/ISC at time 0 and t = minutes. The time to reach half of the maximal ISC (t1/2) was derived as t1/2 = τln2. To determine the functional half-life (t1/2) of ENaC, differentiated HBE cultures were pretreated for 30 minutes with 200 μg/mL CHX or DMSO (vehicle control) and subsequently used for ISC recording. The percentage of maximal INa was plotted over time at 10-minute intervals, and fit to a linear equation (y = y0 + mx). The slope (m) of individual decay plots was corrected to the average slope of untreated control filters, to correct for baseline ISC rundown. The t1/2 was derived as t1/2 = y0/2 m.
Data Analyses and Statistics
Results are expressed as mean ± SE. Significance was determined by unpaired Student's t tests unless otherwise indicated. P < 0.05 was considered statistically different.
RESULTS
Sodium Absorption Increases after ASL Washout in an Ussing Chamber
We and others previously noted that when the ASL volume of airway epithelium is expanded, the transepithelial Na+ absorption increases dramatically (6, 7). This increase was attributed to the washout of substances that inhibit ENaC, such as protease inhibitors or ATP. Na+ and water absorption must increase in response to increased luminal volume, to maintain optimal mucociliary clearance and prevent bronchorrhea. To study this increase in ENaC activity, primary HBE cells were mounted in a well-mixed Ussing chamber, to increase the apical volume dramatically (apical chamber volume, 3 mL), effectively dilute the endogenous inhibitory factors present in the ASL, and perform transepithelial short-circuit current measurements.
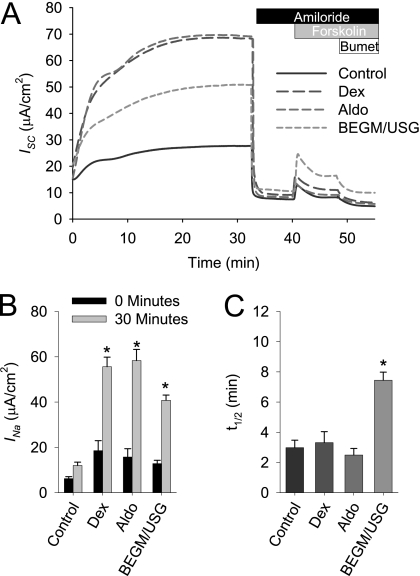
As shown in Figure 1, the short circuit increases after apical washout from 12.8 ± 1.5 μA/cm2 to 40.8 ± 2.3 μA/cm2 over a 30-minute interval, when HBE cells are cultured in differentiation media (BEGM/USG). To assess whether growth factors or steroids increase the expression of ENaC and are necessary for the increase in INa after mounting in an Ussing chamber, HBE cells were incubated overnight in base medium (control) or DMEM containing either 50 nM aldosterone or 50 nM dexamethasone. Without steroids or growth factors, a reduced amiloride-sensitive current was evident, and the increase in INa after the 30-minute equilibration period was modest (initial current, 6.2 ± 0.8 μA/cm2; at 30 minutes, 12.0 ± 1.5 μA/cm2). In the presence of aldosterone or dexamethasone, significant increases in INa occurred during the 30-minute equilibration period (15.7 ± 3.7 to 58.3 ± 4.9 μA/cm2 and 18.5 ± 4.4 to 55.6 ± 4.2 μA/cm2, respectively).
Figure 1.
Na+ absorption increases after airway surface liquid (ASL) washout in an Ussing chamber. Human bronchial epithelial (HBE) cell cultures were cultured in DMEM/F12 ± bronchial epithelial growth medium (BEGM), 50 nM dexamethasone (Dex), or 50 nM aldosterone (Aldo) for 24 hours before short-circuit current (ISC) recording. After a 30-minute equilibration, 10 μM of amiloride were added to the apical chamber to determine the amiloride-sensitive ISC (INa). Subsequently, Cl− secretion was assessed by adding 10 μM forskolin and then 100 μM bumetanide to the basolateral chamber. Data shown are (A) representative ISC recordings, (B) mean amiloride-sensitive current (INa) ± SE, and (C) and mean t1/2 ± SE of the INa increase from 0 to 30 minutes (n = 12 cultures from three tissue donors). USG, Ultroser G. *P < 0.001, different from control culture as determined by ANOVA with Bonferroni post hoc analysis.
In an attempt to elucidate the mechanism behind the increase in Na+ conductance associated with ASL washout, we calculated the kinetics of the rise in current during the initial 30 minutes. The t1/2 of the increase in INa varied between cultures derived from different donors, and therefore these kinetics measurements were performed in parallel, using matched cultures on each individual day of the experiment. In the experiments presented in Figure 1C, the t1/2 of the INa increase during the 30-minute equilibration period under the control, dexamethasone-stimulated, and aldosterone-stimulated conditions was relatively similar. However, the rate of INa increase was significantly slower under BEGM/USG conditions. The expansion of ASL was associated with an increase in INa that occurs over a period of minutes, suggesting that the regulation is not a result of new channel synthesis.
Next, a series of experiments was performed to confirm that the increase in INa observed during ASL washout is attributable to an increase in conductive sodium absorption though ENaC. To ensure that the increase in INa did not reflect an artifact introduced by voltage clamping, HBE cultures were placed into the Ussing chamber, and the transepithelial electric potential difference (VT) was recorded under open-circuit conditions (as shown in Figure E1 in the online supplement). Because the increase in VT is similar to the increase in ISC over the 30-minute interval, the increase in current after placement in the Ussing chamber was unlikely to be the result of voltage clamping. To determine whether the increase in INa after ASL washout was attributable to a change in apical membrane conductance or changes in Na+/K+ ATPase activity, we performed apical and basolateral permeabilization experiments (Figure E2). Interestingly, when the basolateral membrane was permeabilized with nystatin and an apical to basolateral sodium gradient was established, the increase in INa during ASL washout was diminished, suggesting that either an intact plasma membrane is required for the response, or that a change occurred in Na+/K+ ATPase activity during the experiment. Therefore, we directly determined whether Na+/K+ ATPase activity changes during the course of the ASL washout. After apical permeabilization with nystatin, a large increase in ISC was observed, demonstrating that apical membrane conductance constitutes the rate-limiting step in transepithelial Na+ absorption. Because the ISC was abolished with the addition of basolateral ouabain, the peak ISC observed after apical permeabilization was assumed to reflect ATPase activity. No change in ATPase activity was evident during the course of ISC recording and ASL washout. Therefore, the increase in ISC associated with ASL washout is likely attributable to a change in ENaC activity, and not an artifact of voltage clamping or changes in Na+/K+ ATPase activity.
Proteolytic Activation of ENaC in Response to ASL Washout
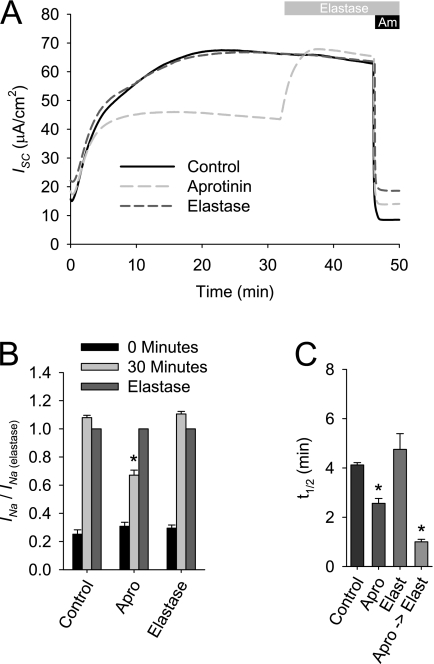
Previous work suggested that the increase in Na+ absorption after ASL volume expansion is attributable to the dilution of soluble protease inhibitors and a subsequent proteolytic activation of ENaC's by CAPs (6, 7). Therefore, we investigated the degree to which the proteolytic activation of ENaC contributes to the observed increase in INa after ASL washout in an Ussing chamber. Matched HBE cultures were mounted in the Ussing chamber containing Ringer's solution, with or without 300 nM elastase or 10 μM aprotinin. This combination of CAP and protease inhibitor was selected because aprotinin was previously shown to inhibit Na+ currents, and elastase is not inhibited by aprotinin (16–18), allowing for the sequential addition of elastase without concerns about protease inhibition. As shown in Figure 2, the ISC of cultures that were exposed to elastase increased at a rate similar to that in control cultures. After the initial equilibration period, elastase treatment had no effect on INa in the control HBE cultures. Under conditions where the serine protease inhibitor aprotinin was included in the apical Ringer's solution, the ISC initially increased at the same rate as in HBE cultures with control Ringer's solution or Ringer's solution containing elastase. However, the steady-state ISC was lower than that under control conditions, resulting in a faster t1/2. In the aprotinin-treated HBE cultures, elastase elicited a rapid increase in ISC, as previously reported (17). Thus, approximately 35% of the increase in INa associated with ASL washout is attributable to the proteolytic activation of inactive channels. Furthermore, the kinetics profile of the INa increase suggests that the proteolytic activation of ENaC is rapid in HBE, and not rate-limiting, during the increase in Na+ absorption after ASL dilution.
Figure 2.
Effects of proteases and protease inhibitors on acute activation of epithelial sodium channel (ENaC) after ASL washout in an Ussing chamber. HBE cultures were mounted in an Ussing chamber containing apical Ringer's solution ± 300 nM elastase (Elast) or 10 μM aprotinin (Apro). After a 30-minute equilibration, 300 nM of elastase were added to the apical chamber. Data shown are (A) representative ISC recordings, (B) mean amiloride-sensitive current normalized to the post-elastase amiloride-sensitive current (INa/INa(elastase)) ± SE, and (C) mean t1/2 ± SE of the INa increase from 0 to 30 minutes (n = 8 cultures from two tissue donors). *P < 0.01, different from control culture as determined by ANOVA with Bonferroni post hoc analysis.
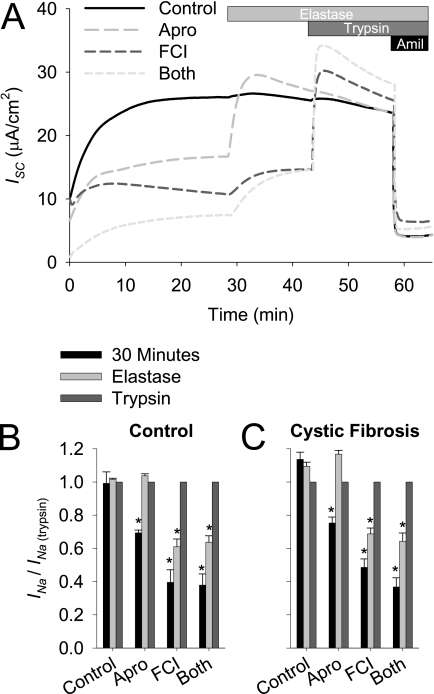
We next investigated whether processing by multiple proteases is required for full ENaC activity in airway epithelia. The mechanism of channel activation by proteases is thought to occur via the removal of inhibitory peptide tracks after double cleavage of the α and γ subunits (18–23). We reasoned that we could manipulate the degree to which the channel is processed by applying various protease inhibitors, and then sequentially adding proteases to activate ENaC fully. To inhibit different protease families, HBE cultures were incubated with apical Ringer's solution, with or without aprotinin to inhibit serine proteases, including those in the CAP family, or a furin convertase inhibitor (FCI) to inhibit pro-protein convertases, such as furin, for 16 hours before ISC recording. After the plateau ISC was achieved, the HBE cultures were sequentially exposed to elastase, which cleaves a single site within the γ subunit (18, 24), and trypsin, which was shown to process the α and γ subunits fully (25). The INa was normalized to the maximal INa observed after trypsin addition (INa/INa(trypsin)) or the maximal ENaC-dependent current. As demonstrated in Figures 3A and 3B, when serine proteases were inhibited by aprotinin, the ISC reached a plateau that was 69.4% ± 1.7% of maximal (P = 0.004, different from control Ringer's solution-treated HBE cultures, n = 12 cultures from three tissue donors). In aprotinin-treated cultures, a single cleavage of the γ subunit was sufficient to restore maximal channel activity, and the subsequent addition of trypsin exerted no further effect. The inhibition of furin and related pro-protein convertases with FCI caused a more pronounced reduction in INa. In contrast to the aprotinin-treated HBE cultures, treatment with elastase only partly restored channel activity in the FCI-treated cultures, and when the other cleavage sites were processed by trypsin, the channel was fully activated. Therefore, multiple cleavage events are required to activate the channel fully when the endogenous pro-protein convertases are inhibited.
Figure 3.
Effect of aprotinin and a furin convertase inhibitor on the acute activation of ENaC in control and cystic fibrosis (CF) HBE cultures. HBE cells from control and CF tissue donors were cultured with apical Ringer's solution and basolateral media, with or without 10 μM aprotinin or 50 μM furin convertase inhibitor (FCI) for 16 hours before ISC recording. After a 30-minute equilibration, 300 nM elastase and 10 μM trypsin were sequentially added apically. Data shown are (A) representative ISC recordings, and (B and C) mean amiloride-sensitive current normalized to the post-trypsin amiloride-sensitive current (INa/INa(trypsin)) ± SE at 30 minutes, after elastase, and after trypsin for (B) control and (C) CF cultures (n = 12 cultures from three tissue donors). *P < 0.004, different from control culture as determined by ANOVA with Bonferroni post hoc analysis.
We and others previously reported that cystic fibrosis (CF) HBE cultures exhibit an imbalance between endogenous CAPs and protease inhibitors, leading to increased levels of proteolytic activation of ENaC in the CF airway (6, 7). Therefore, we tested the susceptibility of HBE cultures from CF tissue donors to protease inhibitors and exogenous CAPs. As shown in Figures 3B and 3C, no differences were evident between CF and control HBE cultures in the response to protease inhibition or activation by aprotinin or FCI. Because these experiments were performed after overnight ASL washout, the lack of difference between CF and control HBE cultures suggests that the difference in proteolytic processing between CF and non-CF HBE cultures is only evident when cells are maintained on an air–liquid interface.
ENaC Half-Life Is Similar between Non-CF and CF HBE Cultures
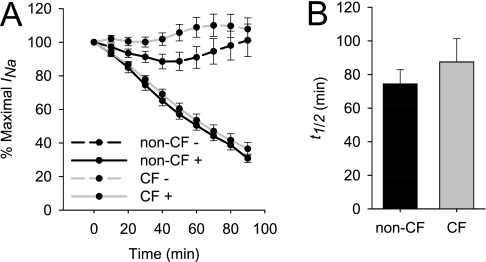
Knight and colleagues previously showed that channels containing the Liddle's mutations undergo increased proteolytic processing as the result of a prolonged membrane residency time (26). Therefore, we questioned whether mutant cystic fibrosis transmembrane conductance regulator (CFTR) prolongs the functional t1/2 of ENaC as a potential means by which CF HBE cells undergo excessive ENaC processing. To determine the functional channel t1/2, CF and non-CF HBE cells were treated with CHX, and the ISC was monitored to determine the decay rate in channel activity, as shown in Figure 4. Interestingly, the increase in INa was not affected by treatment with CHX (Figure 5B), suggesting that new channel synthesis is not required for channel activation with ASL volume expansion. The ISC decreased after treatment with CHX, and the functional t1/2 was determined as described in Materials and Methods. Although variability was observed between tissue donors, no significant differences in ENaC t1/2 were observed between non-CF and CF HBE cultures (74.366 ± 8.539 minutes versus 87.442 ± 13.88 minutes, P = 0.82 according to Mann-Whitney rank sum test, n = 19–22 cultures from five tissue donors). Based on these functional half-life decays, the CFTR genotype appears unlikely to affect the rate at which ENaC is degraded, and no differences in ENaC activity were consistently observed between CF and non-CF HBE cultures when the ASL was expanded.
Figure 4.
Functional ENaC half-life in control and CF HBE cultures. (A) Differentiated non-CF and CF HBE cultures were treated with 200 μg/ml cycloheximide (as indicated by +), and ISC was measured at 10-minute intervals. (B) Functional t1/2 was calculated from the ISC decay for each individual culture. Data shown are mean ± SE, n = 19–22 cultures from five tissue donors.
Figure 5.
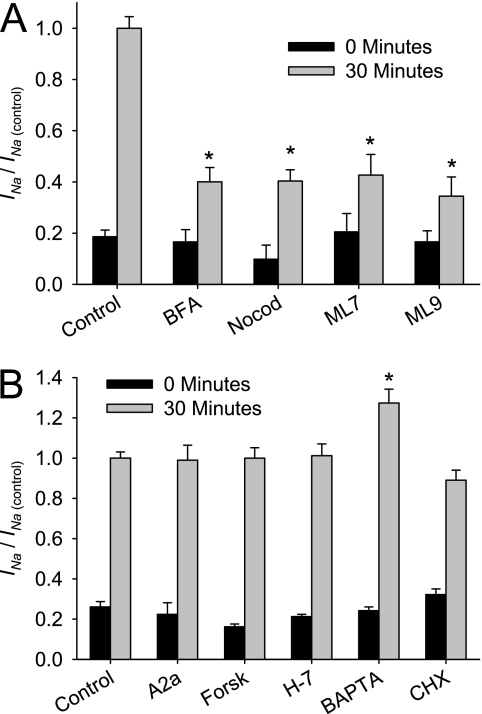
Effect of trafficking inhibition, A2a receptor blockade, BAPTA-AM, cyclic adenosine monophosphate (cAMP), and cycloheximide (CHX) on acute activation of ENaC after ASL washout. Differentiated HBE cultures were exposed basolaterally to the indicated drug for 30 minutes before ISC recording and in Ringer's solution bath during ISC recording. (A) To determine the contribution of membrane trafficking during ISC recording, 5 μg/ml brefeldin A (BFA), 10 μM nocodazole (Nocod), 100 μM ML-7, or 100 μM ML-9 were added to inhibit trafficking. (B) The effects of A2a (A2a), 10 μm forskolin (Forsk), 50 μM H-7, or 10 μM BAPTA-AM were also examined. After a 30-minute equilibration, 10 μM of amiloride were added to the apical chamber. Data shown are the mean amiloride-sensitive current normalized to the mean amiloride-sensitive current of matched control HBE cultures after the 30-minute equilibration (INa/INa(control)) ± SE at 0 minutes (black) and 30 minutes (gray) (n ≥ 6 cultures from ≥2 tissue donors). *P < 0.001, different from control culture as determined by ANOVA with Bonferroni post hoc analysis.
ENaC Traffics to the Apical Membrane in Response to ASL Washout
The results of protease inhibitor experiments suggest that ∼ 60% of channels are in an active state. Because the increase in INa associated with ASL washout is larger than could be accounted for by protease activation, we investigated whether a trafficking event of ENaCs to the cell surface occurs in response to ASL washout. HBE cultures were preincubated with a variety of trafficking inhibitors for 30 minutes before ISC recording. The amiloride-sensitive currents at 0 and 30 minutes of treated HBE cultures were normalized to the INa of untreated control cultures (INa/INa(control)). As shown in Figure 5A, the recorded ISC from HBE cultures that were pretreated with BFA, which disrupts ENaC trafficking from the trans-Golgi network to the apical membrane, was significantly smaller than in control cells, and increased to 40.1% ± 5.6% of the amiloride-sensitive current of control cultures after the 30-minute equilibration period. Similar results were evident after microtubule disruption with nocodazole and with myosin light chain kinase inhibition (ML-7 and ML-9), yielding 40.4% ± 4.4%, 42.7% ± 8.1%, and 34.5% ± 7.4% of the amiloride-sensitive ISC of control HBE cultures at 30 minutes, respectively. Therefore, when trafficking is disrupted though a variety of methods, the ability of HBE cultures to increase sodium absorption in response to ASL volume expansion is impaired. These results suggest that a trafficking step is likely involved with the increase in INa in response to ASL washout.
To support the electrophysiologic results suggesting that ENaC is trafficked in response to ASL expansion, we sought to obtain corroborating biochemical evidence. To identify a suitable ENaC antibody, we tested the ability of numerous commercially available antibodies to immunoblot (IB) and immunoprecipitate (IP) human ENaCs derived from cells transiently transfected with epitope-tagged ENaCs (data not shown). Although some ENaC antibodies targeting the N-terminal region of α ENaC were capable of IP or IB, we were unable to obtain consistent biochemistry in the biotinylated materials. We speculate that this difficulty was a result of limited access of biotin to the short N-extracellular segment of cleaved α ENaC.
Next, to identify the stimulus that initiates trafficking in HBE cultures and other potential mechanisms of ENaC regulation involved in the increase of INa in response to ASL expansion, we tested the effects of a panel of chemical inhibitors that were shown to affect ENaC activity. These compounds were added to the basolateral media for 30 minutes before ISC recording, and were included in the Ringer's solution bath when cells were mounted in Ussing chambers, specifically to inhibit membrane receptors or block secondary messenger cascades. The amiloride-sensitive current was measured at 0 and 30 minutes, and normalized to that of matched, untreated control cultures (INa/INa(control)). As shown in Figure 5B, INa was not altered by the A2a receptor blockade, cAMP activation by forskolin, PKA/PKC inhibition by H-7, Ca+ chelation by BAPTA-AM, or the prevention of new channel synthesis by CHX. Therefore, although the A2a receptor, cAMP, PKA/PKC, and Ca+ signaling may be important for ENaC regulation under some conditions, they do not play a significant role in the acute regulation of ENaC in HBE cells after ASL expansion.
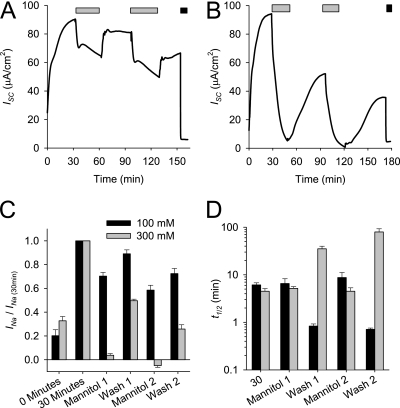
Willumsen and colleagues demonstrated that luminal hypertonicity decreases Na+ absorption across cultured airway epithelia (27). The effects of osmolarity on ENaC activity were investigated to determine whether the INa change after osmolarity shifts is consistent with a trafficking event. As demonstrated in Figure 6, the ISC of differentiated HBE cultures was measured during the sequential osmolarity shifts induced by the addition and removal of 100 mM or 300 mM mannitol to the Ringer's solution bath. After the addition of 100 mM mannitol, the ISC decreased rapidly, and this trend reversed when the mannitol was removed. Similar responses were observed with 300 mM mannitol, although the magnitude of response was larger, and the ISC recovery was slower and incomplete. Because the majority of recorded ISC in HBE is amiloride-sensitive (Figure 1), these ISC changes in response to osmolarity shifts likely reflect changes in ENaC activity. Therefore, luminal osmolarity reversibly affects the INa with kinetics that are consistent with trafficking events, suggesting that ASL osmolarity regulates the number of ENaCs at the cell surface.
Figure 6.
Effect of apical osmolarity on INa. Representative ISC tracings of differentiated HBE cultures during the addition (light gray bar) and removal of 100 mM (A) and 300 mM (B) mannitol. Amiloride was applied (black bar) at the end of the experiment to determine the INa. (C) INa relative to INa at 30 minutes during the various timeouts during the experiments with 100 mM mannitol (black bar) and 300 mM mannitol (gray bar). Data shown are mean ± SE amiloride-sensitive current normalized to the INa at 30 minutes (n = 5–11 HBE cultures). (D) Kinetics of INa changes associated with osmolarity shift induced by 100 mM mannitol (black bar) and 300 mM mannitol (gray bar). Data shown are mean ± SE t1/2, as defined in Materials and Methods (n = 5–11 HBE cultures).
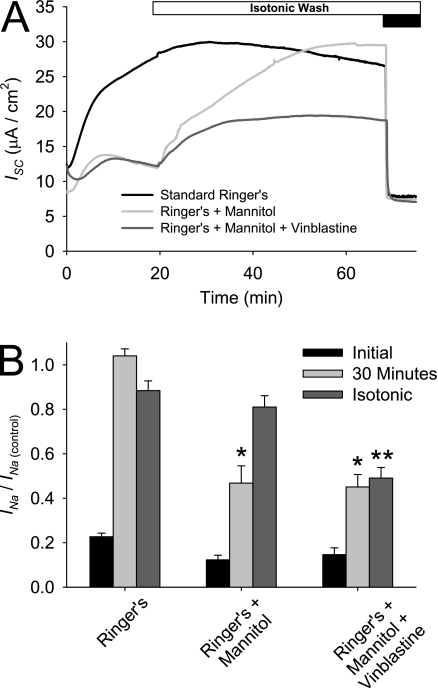
We next investigated whether the trafficking event responsible for the increase in INa during ASL washout could be modulated by the osmolarity of apical Ringer's solution. Differentiated HBE cultures, with and without pretreatment with 50 μg/mL vinblastine to disrupt tubulin, were mounted in an Ussing chamber in which the apical Ringer's solution bath contained 100 mM mannitol. As demonstrated in Figure 7A, the bath solution was replaced with symmetric, isotonic Ringer's solution after the initial 30-minute equilibration. The amiloride-sensitive current at 0 minutes, at 30 minutes, and after the isotonic wash was normalized to that of matched, untreated control cultures (INa/INa(control)). After the initial 30-minute equilibration, a large increase in ISC was evident under control conditions. This ISC increase was significantly smaller when mannitol was included in the apical Ringer's solution (46.8% ± 7.7% and 45.1% ± 5.6% of the control INa for mannitol and mannitol + vinblastine conditions, respectively). After replacement of the hypertonic bath solution with isotonic Ringer's solution, the INa increased and matched that of control cultures. The observed increase in INa after the isotonic wash was prevented when HBE cells were pretreated with vinblastine, suggesting that trafficking and not changes in ENaC PO are responsible for the INa increase after the osmolarity shift. These results suggest that the increase in INa after ASL washout is partly attributable to a change in osmolarity of the apical fluid and a resultant trafficking event.
Figure 7.
Effect of hyperosmolarity and trafficking inhibitors on INa during ASL washout. Differentiated HBE cultures were incubated basolaterally, with and without 50 μg/mL vinblastine for 30 minutes before ISC recording. The apical Ringer's solution during the initial portion of the ISC trace was isotonic or contained an additional 100 μM mannitol. After the initial equilibration period, bathing solutions were exchanged with several washes of isotonic Ringer's solution. Amiloride was applied (black bar) at the end of the experiment to determine the INa. Data shown are (A) representative ISC recordings, and (B) mean amiloride-sensitive current normalized to the amiloride-sensitive current of control HBE cells at 30 minutes (INa/INa(control)) ± SE at 0 minutes, at 30 minutes, and after the isotonic wash (n > 12 cultures from two tissue donors). *P < 0.01, different from control culture as determined by ANOVA with Bonferroni post hoc analysis.
DISCUSSION
To allow for increased fluid absorption when the ASL volume is expanded, Na+ absorption via ENaC increases in two ways, though trafficking of channels to the cell surface to increase channel density, and by proteolytic activation of incompletely processed channels to augment Na+ conduction through membrane–resident channels. After ASL expansion, ENaC trafficking to the luminal surface accounts for approximately 60% of the acute increase in INa. Proteolytic activation occurs rapidly, and accounts for the remainder of the increase in INa. Previous work demonstrated that this proteolytic activation occurs as a result of dilution of soluble protease inhibitors, allowing for endogenous CAPs to process the channel rapidly (6, 7). The activation kinetics suggest that ENaC activity in the airway is modulated by both proteases and trafficking events.
The proteolytic regulation of ENaC involves multiple proteases and multiple cleavage sites within the subunits' extracellular domains. As predicted by work conducted in oocyte expression systems, more than one cleavage event is required to activate ENaC fully (18–23). During the channel's biogenesis, the pro-protein convertase furin cleaves and activates the channel through a limited proteolysis of α ENaC at two sites within the extracellular domain and one site on γ ENaC (19). Channels that are processed by furin display a moderate PO, whereas uncleaved channels have a low PO (28). The γ subunit undergoes additional processing on the cell surface by prostasin (21), elastase (18, 24), and plasmin (22, 29), which leads to maximal channel activation. Based on work in heterologous systems, proteolytic processing is postulated to activate ENaC via the removal of inhibitory peptide domains that span the two cleavage sites within the extracellular domain (23). Our results suggest that multiple cleavage events are also required for full channel activation in HBE, which endogenously expresses ENaCs.
Because of the paucity of reliable antibodies that detect the cleaved forms of endogenously expressed ENaC, it is difficult to determine which subunits are processed, and to what extent cleavage occurs when the ASL is expanded. However, aprotinin appears to inhibit CAPs that activate ENaC via cleavage of the γ subunit, because elastase, which only cleaves the γ subunit (18, 24), is capable of fully activating the channel in aprotinin-treated cultures. When additional proteases were inhibited with FCI, the single cleavage event caused by elastase was insufficient to restore full channel activity. Restoration of normal channel activity in FCI-treated cultures required the addition of trypsin, and it is unclear if this additional activation is attributable to further processing of the α or γ subunit. Recent work from Carattino and colleagues suggests that the relevant activating proteolysis occurs with double cleavage of the γ subunit and removal of the intervening inhibitory peptide, regardless of the degree to which α is processed (30). It may be possible to address these questions biochemically in the future, when validated high-quality ENaC antibodies become available.
Several lines of evidence suggest that ENaC trafficking contributes to the increased INa with ASL expansion. First, the addition of elastase to the apical bathing solution from the beginning of recording in the Ussing chamber demonstrates that the rate of INa activation after ASL expansion is unaltered by the presence of exogenous protease or protease inhibitor. Because the rate of channel activation by elastase is more rapid than the rate of INa increase with ASL expansion, the addition of elastase to the apical surface would be expected to accelerate the ISC increase, if unprocessed channels were present in the apical membrane. We observed a lack of effect, suggesting that channels residing in the apical membrane under basal conditions are fully processed, and that activation by CAPs is not rate-limiting. Furthermore, when endogenous CAPs were inhibited by aprotinin and the HBE cultures were subsequently exposed to elastase, a pool of protease-susceptible channels is evident after 30 minutes. This finding suggests that these elastase-activated channels represent uncleaved ENaCs that were delivered from an elastase-inaccessible subapical pool. Otherwise, they would have been activated when elastase was included at the beginning of the trace. Second, blocking trafficking though a variety of different means significantly attenuated the increase in INa after ASL expansion, suggesting the either ENaC or an accessory protein is trafficked with ASL expansion. Therefore, our findings suggest for the first time, to the best of our knowledge, that regulated channel trafficking is an additional means by which airway epithelia regulate the ASL volume.
The signaling cascade responsible for initiating the trafficking response was not completely resolved in these studies. Although an increase in apical osmolarity clearly and significantly inhibited the increase in INa observed with ASL expansion, pharmacologic inhibitors of a number of potential pathways had no effect on the INa increase. The localized sensing of changes to ASL osmolarity and the ability to direct ENaC to the apical surface in response to a hyposmotic challenge would represent a cellular solution for maintaining ASL homeostasis, and requires further investigation.
In this study, no difference in ENaC susceptibility to protease inhibitors and functional channel half-life between non-CF and CF HBE cultures was observed. In our previous report (6), we observed an increased inhibition of ENaC by aprotinin and decreased activation by trypsin in CF HBE cells that were cultured at basal ASL volumes, demonstrating an excessive proteolytic activation of ENaC in CF epithelia. In the present study, when the ASL volume was expanded, the difference in the protease–antiprotease balance between CF and non-CF was not apparent, suggesting that these differences are only apparent under thin film conditions. Furthermore, in our previous report, a large increase in INa was evident after the addition of trypsin, whereas here, the addition of elastase had no effect on INa. One potential source for this difference involves the use of a different Ussing chamber, whose contents were well-mixed by the flow established by gassing the chamber. This increased flow likely prevents the retention of an unstirred layer at the apical surface, whereas the apical surface was relatively undisturbed in the chamber used in the previous study. Our results suggest that the INa results obtained from airway epithelia in a well-mixed Ussing chamber are dramatically altered by the removal of endogenous ASL, and may not accurately reflect the physiology of the in vivo airway under physiologic conditions. Therefore, studying the physiologic regulation of airway ENaCs in standard Ussing chamber systems is difficult, and alternative approaches, such as the “thin film” approach used by Tarran and Boucher (31), may be more suitable for studying airway epithelial electrophysiology.
In conclusion, sodium absorption across airway epithelia increases when the ASL volume is expanded by the coordinated trafficking of a reserve pool of channels to the cell surface and subsequent proteolytic activation. This reserve pool of channels consists of partly processed channels that, when trafficked to the cell surface, increase INa to approximately two thirds of the maximal current. For maximal activation, the channel must be processed by CAPs that are present in abundance on the cell surface, and that are highly active when endogenous inhibitors in the ASL are diluted or washed out.
Acknowledgments
The authors thank the Lung Transplantation Program at the University of Pittsburgh Medical Center for facilitating tissue acquisition, and Joseph Latoche for technical assistance.
This work was supported by grants from the National Institutes of Health (K08 HL087932 to M.M.M., P30 DK072506 to J.M.P., and K99-DK07891 to M.B.B.) and from the Cystic Fibrosis Foundation (MYERBU07Q0 to M.M.M., R883 to J.M.P., and BUTTER06GO to M.B.B.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0348OC on January 22, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998;95:1005–1015. [DOI] [PubMed] [Google Scholar]
- 2.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest 1998;102:1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol 2001;118:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J Clin Invest 2000;105:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest 2007;132:1631–1636. [DOI] [PubMed] [Google Scholar]
- 6.Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hypersabsorption in cystic fibrosis. J Biol Chem 2006;281:27942–27949. [DOI] [PubMed] [Google Scholar]
- 7.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 2006;127:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong Z, Illek B, Bhagwandin VJ, Verghese GM, Caughey GH. Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol 2004;287:L928–L935. [DOI] [PubMed] [Google Scholar]
- 9.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na(+) channels are regulated by flow. Am J Physiol Renal Physiol 2001;280:F1010–F1018. [DOI] [PubMed] [Google Scholar]
- 10.Fronius M, Clauss WG. Mechano-sensitivity of ENaC: may the (shear) force be with you. Pflugers Arch 2008;455:775–785. [DOI] [PubMed] [Google Scholar]
- 11.Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol 1999;276:C827–C837. [DOI] [PubMed] [Google Scholar]
- 12.Mall M, Wissner A, Gonska T, Calenborn D, Kuehr J, Brandis M, Kunzelmann K. Inhibition of amiloride-sensitive epithelial Na(+) absorption by extracellular nucleotides in human normal and cystic fibrosis airways. Am J Respir Cell Mol Biol 2000;23:755–761. [DOI] [PubMed] [Google Scholar]
- 13.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredburg JJ, et al. Normal and cystic fbrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem 2005;280:35751–35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 1997;77:359–396. [DOI] [PubMed] [Google Scholar]
- 15.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 2000;279:C461–C479. [DOI] [PubMed] [Google Scholar]
- 16.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 1997;389:607–610. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 2005;288:L813–L819. [DOI] [PubMed] [Google Scholar]
- 18.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of {gamma} ENaC mediates elastase activation of Na+ transport. J Gen Physiol 2007;130:611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 2004;279:18111–18114. [DOI] [PubMed] [Google Scholar]
- 20.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem 2006;281:18901–18907. [DOI] [PubMed] [Google Scholar]
- 21.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma subunit. J Biol Chem 2007;282:6153–6160. [DOI] [PubMed] [Google Scholar]
- 22.Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 2009;20:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 2009;284:20447–20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem 2007;282:58–64. [DOI] [PubMed] [Google Scholar]
- 25.Knight KK, Wentzlaff DM, Snyder PM. Intracellular sodium regulates proteolytic activation of the epithelial sodium channel. J Biol Chem 2008;283:27477–27482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight KK, Olson DR, Zhou R, Snyder PM. Liddle's syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci USA 2006;103:2805–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willumsen NJ, Davis CW, Boucher RC. Selective response of human airway epithelia to luminal but not serosal solution hypertonicity. Possible role for proximal airway epithelia as an osmolality transducer. J Clin Invest 1994;94:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 2006;290:F1488–F1496. [DOI] [PubMed] [Google Scholar]
- 29.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem 2008;283:36586–36591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem 2008;283:25290–25295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarran R, Boucher RC. Thin-film measurements of airway surface liquid volume/composition and mucus transport rates in vitro. Methods Mol Med 2002;70:479–492. [DOI] [PubMed] [Google Scholar]