Abstract
Epigenetic regulation of chromatin is dependent on both the histone protein isoforms and state of their post-translational modifications. The assignment of all post-translational modification sites for each individual intact protein isoform remains an experimental challenge. We present an on-line reversed phase LC tandem mass spectrometry approach for the separation of intact, unfractionated histones and a high resolution mass analyzer, the Orbitrap, with electron transfer dissociation capabilities to detect and record accurate mass values for the molecular and fragment ions observed. From a single LC-electron transfer dissociation run, this strategy permits the identification of the most abundant intact proteins, determination of the isoforms present, and the localization of post-translational modifications.
Over the last two decades, the integration of the unanticipated discovery of new modes of internal energy deposition and rapid technological progress in mass spectrometry-based instrument platforms has revolutionized our experimental capabilities to effectively study the inherent complexity of human and other mammalian proteomes. This includes the rapid identification of proteins, the detection and unambiguous assignment of protein isoforms, and the detection and localization of post-translational modifications (PTMs).1
The vast majority of mass spectrometry-based studies in protein biology and proteomics has used proteolytic digestion using trypsin and analysis of the resulting peptide mixtures by a variety of MS and MS/MS approaches (1–4). Although of considerable use for many purposes, this common strategy has a variety of limitations especially from the view of acquiring the information needed to establish and understand biological function. These limitations include the loss of information regarding the nature of protein isoforms and a loss of knowledge of the global presence of multiple PTMs that can co-occur at differing sites on the same protein molecule. These deficiencies are exacerbated when the portions of protein sequence coverage observed in a digest are low because peptides that would reveal the modifications or isoform-specific sequence variances may not have been detected. Additionally, the sequence identification for fragment ion spectra representing peptides with modifications that are unanticipated can be difficult to assign using common search algorithms.
Hence, structural information covering an entire protein sequence has been lacking, and although needed from a protein biology viewpoint all along, the kind of methodology that would be required to obtain this knowledge has begun to emerge only recently. This has come about through the discovery of electron capture by polyprotonated peptide and protein species (5) and the more recent development of electron transfer by their reaction with suitable radical anions (6). Because these physicochemical processes deposit sufficient internal energy at the sites of charge reduction to cleave peptide backbone bonds promptly (non-ergodically), the distribution of cleavage sites reflects the potential positions of protonation along the peptide/protein backbone without the usual redistribution of vibronic energy that occurs following collisional activation. Thus, these energy deposition techniques are being exploited in Fourier transform-ion cyclotron resonance (7, 8) and Orbitrap platforms (9) and are being developed further to provide access to fragmentation information on peptides significantly larger than those derived by tryptic digestion and smaller proteins even on a chromatographic time scale.
As noted above, these powerful new fragmentation techniques are able to generate a greater frequency of protein backbone cleavages even as peptide/protein size is increased by more than a factor of 100 over tryptic peptides. In addition, labile covalent modifications are generally retained because internal energy randomization is minimal (10, 11). These properties of electron capture dissociation (ECD) and electron transfer dissociation (ETD) have provided the experimental basis (12–14) for the rigorous analysis of peptides/proteins bearing variable combinations of multiple PTMs for the first time, providing revolutionary advantages of particular importance for many challenges in the characterization of biological systems.
One class of small highly basic proteins seems to provide a virtually ideal test bed for further development of these methods, namely the histones. Histones are the archetypical class of chromatin regulatory and packaging substances bearing a yet to be determined complex variety of reversible post-translational modifications, including but not limited to acetylation, methylation, ubiquitination, and phosphorylation. Even the exact same protein sequence may be modified by multiple PTMs in varying combinations and levels of site occupancy. There are over 60 residues on the four core histones subject to one or more reversible modifications by the small moieties noted above (15–20). These proteins are not only integral components of chromatin and the nature of its diverse functional repertoire but play a highly complex dynamic role in virtually all cellular processes. It is hypothesized that particular combinations of PTMs embody a histone code that is recognized by effector proteins (mostly still unknown) that are recruited specifically to modified histones to carry out essential functional processes on chromatin (21). Disruption of these sites and modifications and/or the enzymatic processes that regulate particular occupancy states is intimately associated with a wide variety of human diseases from developmental diseases to cancer (22). Thus, gaining an understanding of the processes controlling the modulation of chromatin structure and its exquisite differential accessibility by myriad enzymatic activities by the covalent code written on members of the family of histones is a major challenge of our time. Recently, even ε-amino methyl reversibility has emerged through the discovery of demethylases that operate with precise site and methylation state specificity (23).
Historically the chromatin field has relied on immunochemical methodology to probe structure-function studies for many years. Only recently has mass spectrometry begun to play an important role in identifying the types, combinations, specific sites, and abundance of PTMs (15, 16). Despite recent contributions from several laboratories, comprehensive structural characterization of histone modification patterns remains a major analytical challenge. As a result, efforts aimed at facilitating and gaining a comprehensive characterization of multiple post-translational site occupancies are an important pursuit that will accelerate progress in revealing a fundamental knowledge of structural/functional relationships.
Here, we describe progress on intact protein analysis by on-line LC-ESI Orbitrap ETD-MS/MS of unfractionated histone mixtures. These studies provide information on protein identification, protein isoform determination, and localization of PTMs for the most abundant species with minimal sample preparation. We also show that more detailed PTM occupancy information can be obtained from longer sequences derived by Asp-N digestion.
EXPERIMENTAL PROCEDURES
Sample Preparation
Mouse embryonic stem cells were grown in Dulbecco's minimum essential medium supplemented with 5% fetal bovine serum. Cells were harvested by trypsinization and centrifugation at 1500 rpm with two PBS washes. The nuclei were collected as described previously (24) except the cells were resuspended in nuclear isolation buffer containing 20 mm HEPES (pH 7.4), 1.5 mm MgCl2, 10 mm KCl, 0.5 mm DTT, 0.1% Triton, 10 mm sodium butyrate, 2 mm PMSF, 1× phosphatase inhibitor mixture, 1× protease inhibitors (pepstatin, aprotinin, E-64, and leupeptin) (Sigma). Cells were lysed using Dounce homogenization on ice, and nuclei were pelleted at 1200 rpm for 5 min at 4 °C and washed twice with nuclear isolation buffer. Nuclei were resuspended in Triton extraction buffer (PBS, 10 mm sodium butyrate, 0.5% Triton, 2 mm PMSF, 1× phosphatase inhibitor mixture, 1× protease inhibitors) and washed twice with Triton extraction buffer. Histones were then extracted into 0.4 n H2SO4 at 4 °C for an hour with constant rotation. The sample was centrifuged at 14,000 × g for 15 min, the supernatant was collected, and the histones were precipitated with 20% trichloroacetic acid (v/v, final) and incubated for 1 h at −20 °C. The precipitated histones were pelleted at 14,000 × g for 15 min at 4 °C, and the pellets were washed once with ice-cold acetone containing 0.1% HCl and once with ice-cold acetone alone. The protein pellet was then dried, resuspended in water, and stored at −20 °C. Histone samples were analyzed directly by mass spectrometry (intact protein analysis) or following digestion with endoproteinase Asp-N (Roche Applied Science) (1:20) at pH 8 for 4 h.
On-line LC-ESI Orbitrap ETD of Intact Histones
Approximately 45 pmol of total, unfractionated histones were separated by reversed phase chromatography on line with a Thermo LTQ-Orbitrap mass analyzer by use of a Waters nanoACQUITY ultraperformance LC (UPLC) system with a 75-μm-inner diameter × 250-mm-long C18 column (300-Å pore size) packed with 1.7-μm-diameter particles (Waters). The histones were separated using a gradient of 10–40% solvent B over 55 min (solvent A, 0.1% formic acid in water; solvent B, 0.1% formic acid in 100% acetonitrile) at a flow rate of 200 nl/min. The eluate was detected directly by mass spectrometric analysis. Mass spectrometry data were acquired on a hybrid LTQ-Orbitrap equipped with ETD (Thermo). After a precursor scan of the molecular ions was measured in the Orbitrap by scanning from m/z 400 to 1200, the two most intense multiply charged precursors were selected for ETD analysis in the Orbitrap. Both the molecular and the fragment ions were detected in the Orbitrap with a resolution of 60,000 (at m/z = 400). Molecular ions for fragmentation were isolated with a 10 m/z isolation window and 50-ms ETD activation time for fragmentation. The automatic gain control target value for the fluoranthene anions was 2 × 105 with a maximum injection time of 500 ms. The ion/ion reactions were conduction in the linear ion trap before being sent to the Orbitrap for high resolution m/z analysis. The ETD spectra were acquired with an automatic gain control target value of 3 × 105, and each scan included two microscans with an FTMS maximum injection time of 500 ms.
On-line LC-ESI Orbitrap ETD of Asp-N Histone Digests
Unfractionated histone Asp-N digests were analyzed by mass spectrometry as above except the peptides were separated using a two-step gradient of 2–20% solvent B over 35 min followed by a 5-min gradient from 20 to 50%. After a precursor scan of the molecular ions was measured in the Orbitrap by scanning from m/z 350 to 1200, the four most intense multiply charged precursors were selected for ETD analysis in the Orbitrap. The molecular and the fragment ions were detected in the Orbitrap with a resolution of 30,000 and 15,000 (at m/z = 400), respectively. Molecular ions for fragmentation were isolated with a 3 m/z isolation window and 200-ms ETD activation time for fragmentation.
Data Analysis
Data were analyzed using a combination of Xtract in Qual Browser version 2.0 (Thermo Scientific) and two in-house softwares, Protein Prospector and Fragment Assignment by Visual Assistance (FAVA) (25). Xtract was used for the deconvolution of the molecular ion and for peak list generation for the fragment ion spectra. As ion transient data are unavailable on the Orbitrap, each fragment ion spectrum used was an average spectrum composite of multiple fragment ion spectra of the same protein species across the chromatographic peak (ranging from two to five MS/MS spectra). As detailed in the data acquisition settings above, each individual spectrum is the average of two ion transients (microscans) each. These data were then entered into Protein Prospector version 5.3.2 (26), and a search was performed against the Mus musculus entries in the UniProtKB database (downloaded on July 7, 2009 with 60,970 entries). Precursor and fragment mass tolerances of 30 ppm were allowed. Considered modifications were protein N-terminal acetylation, loss of N-terminal methionine, (mono-, di-, and tri-) methylation of lysine residues, acetylation of lysine residues, and phosphorylation of serine and threonine residues. Initial acceptance criteria required a reported expectation value of less than 0.1. All results were then manually verified. Additional undefined mass modification searches were performed; however, no unexpected proteins were identified. Determination of all protein identifications/isoforms and thorough localization of PTMs were performed using FAVA software (25) to accurately assign the fragment ions. The ion assignments, including those used for determination of sites of post-translational modifications, were manually verified. For quantitation of the variably modified histone H4 N-terminal tail peptides, the chromatographic peak areas (following Asp-N digestion) were summed, and the relative abundance of each modified peptide was determined as the average percentage of the total for three biological replicates.
RESULTS
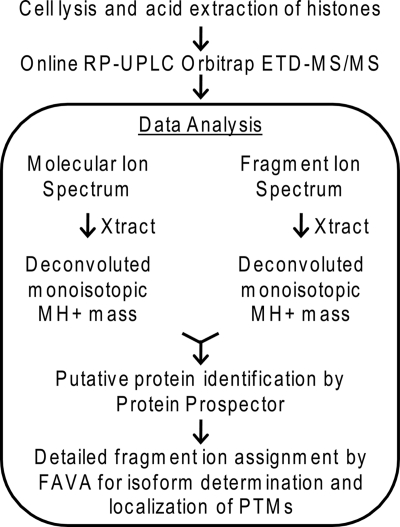
Histones were acid-extracted from mouse embryonic stem cells. This total histone isolate was subjected to separation by high pressure RP C18 chromatography with controlled particle stationary phase and mass spectrometric analysis using ETD on an electrospray LTQ-Orbitrap. The high mass resolution data set was analyzed using a combination of two in-house software packages, Protein Prospector (version 5.3.2) and FAVA (25) (Fig. 1).
Fig. 1.
Protein identification work flow for sample preparation and data analysis for determination of protein isoform and localization of PTMs.
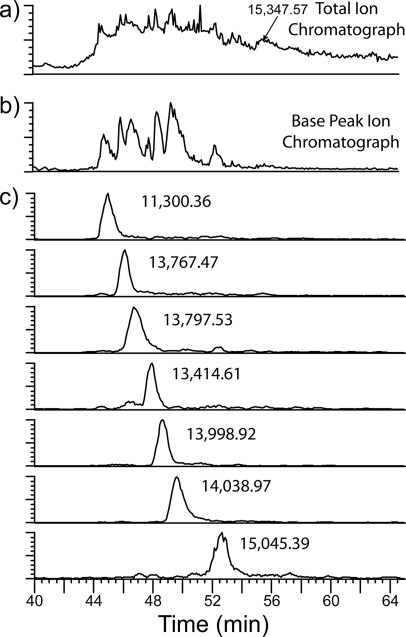
The core histones, histones H2A, H2B, H3, and H4, eluted over ∼15 min (Fig. 2a). The base peak chromatogram (Fig. 2b) shows that the separation achieved resolved approximately seven histone molecular species with elution time profiles in the range of 1–2 min as shown in the extracted ion chromatograms of the most abundant species in each chromatographic peak (Fig. 2c). For each chromatographic peak, the protonated monoisotopic molecular masses of the most abundant species are indicated (Fig. 2c and Table I) using the Xtract software based on THRASH (27). Because of the highly modified nature of the many histone H3 isoforms present, the ion intensity is dispersed across a multitude of variably modified histone H3 species so that the base peak intensities for the molecular ions appear low compared with those observed for the H4, H2B, and H2A isoforms. These H3 variants eluted between 54 and 58 min as indicated in the total ion chromatogram (Fig. 2a) where regions of high total ion abundance are observed centered around 54, 56, and 57.5 min. In addition, the isotopic envelopes of each of the modified species are overlapping as the modified species are not chromatographically resolved. For these reasons, the Xtract software was unable to deconvolute the molecular ion spectra with the exception of the peak at 56 min where the most abundant ion was determined to be 15,347.56 as indicated in the total ion chromatogram in Fig. 2a and listed in Table I.
Fig. 2.
On-line RP UPLC separation of intact, unfractionated histones. a, total ion chromatogram; b, base peak ion chromatogram; and c, extracted ion chromatograms for the most intense ion in each chromatographic peak. The deconvoluted, monoisotopic masses are indicated for the most abundant species in each peak.
Table I. Summary of intact, unfractionated histone analysis by on-line high resolution ETD for protein identification, isoform determination, and localization of PTMs.
| Protein isoform | Accession no. | PTM(s) | MH+ measured | MH+ calculated | Error | Total fragment ions detected | Unique N-terminal fragments detected | Unique C-terminal fragments detected | Fragment ion coverage |
|---|---|---|---|---|---|---|---|---|---|
| Da | Da | ppm | % | ||||||
| Histone H4 | P62806 | N-terminal Met loss, N-terminal acetylation, Lys-20 dimethylation | 11,300.3638 | 11,300.3904 | −2.35 | 129 | 22 | 46 | 67 |
| Histone H2B type 1-C | Q6ZWY9 | N-terminal Met loss | 13,767.4734 | 13,767.5243 | −3.70 | 115 | 25 | 40 | 52 |
| Histone H2B type 1-F | P10853 | N-terminal Met loss | 13,797.5289 | 13,797.5349 | −0.43 | 165 | 32 | 45 | 62 |
| Histone H2A.Z | P0C0S6 | N-terminal Met loss | 13,414.6098 | 13,414.5188 | 6.78 | 73 | 23 | 29 | 41 |
| Histone H2A type 2A | Q6GSS7 | N-terminal Met loss, N-terminal acetylation | 13,998.9218 | 13,998.867 | 3.91 | 122 | 25 | 38 | 49 |
| Histone H2A type 1 | P22752 | N-terminal Met loss, N-terminal acetylation | 14,038.9656 | 14,038.9273 | 2.73 | 130 | 26 | 39 | 50 |
| Histone H2A.X | P27661 | N-terminal Met loss, N-terminal acetylation | 15,045.3945 | 15,045.4132 | −1.24 | 47 | 18 | 17 | 25 |
| Histone H3.3 | P84244 | N-terminal Met loss, Lys-9 dimethylation | 15,347.5715 | 15,347.5414 | 1.96 | 80 | 29 | 21 | 37 |
Intact Histone Identification
A summary of the proteins identified by on-line RP UPLC Orbitrap ETD of unfractionated histones is provided in Table I. The specific isoform, modifications, and sites of modifications are listed where applicable. In all cases, the parent masses were detected within 7-ppm mass error. The ETD fragment ions detected were sufficient to determine the histone isoform and localize the sites of PTMs. It is important to note that although these are the most abundant ions present they are not the only species present. As described above, multiple histone H3 isoforms are present; however, the ability to determine the monoisotopic mass as well as to detect and isolate the molecular ion in a data-dependent fashion is hindered because of the large number of modifications and lack of their separation with RP LC.
Use of ETD Fragment Ions for Determination of Isobaric Histone H2B Isoform
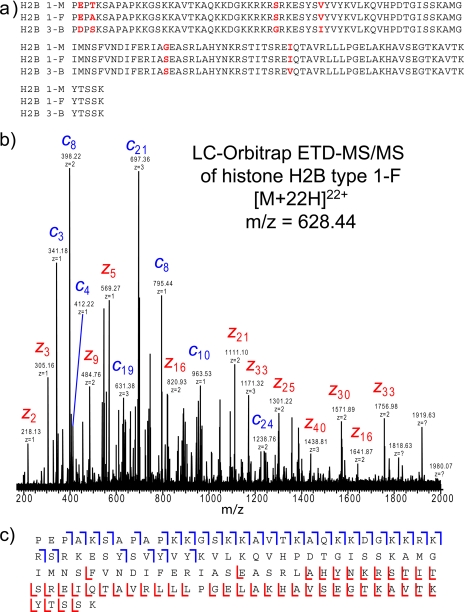
The measured MH+ parent mass of 13,797.5289 Da for the third peak in the extracted ion chromatogram (Fig. 2c) corresponds to three possible histone H2B variants, all with a mass of 13,797.5349 Da (−0.431-ppm mass error): histone H2B type 1-F with a loss of a methionine residue, histone H2B type 3-B with a combined loss of a methionine residue and addition of two methyl moieties, and histone H2B type 1-M with a loss of a methionine residue. The sequence alignment for these three histone H2B sequence variants is shown in Fig. 3a. Interpretation of the on-line ETD fragment ion spectrum enabled the conclusive assignment of histone H2B type 1-F based on the presence of 62% corresponding fragment ion coverage, which includes 30 unique c ions (Fig. 3, b and c). In addition, no ions were detected that could uniquely identify the histone H2B variants, type 1-M or type 3-B, suggesting that only the histone H2B type 1-F isoform is present.
Fig. 3.
Determination of histone H2B isoform by on-line LC Orbitrap ETD-MS/MS analysis. a, sequence alignment for mouse histone H2B types 1-M, 1-F, and 3-B. Sites of sequence variation are indicated in red. b, ETD fragment ion spectrum with only the most abundant ions labeled. The spectrum is an average spectrum composite of three MS/MS spectra. c, fragment ion assignments for histone H2B type 1-F. Although three isobaric H2B variants exist, ETD fragmentation enables the detection and confident identification of histone H2B type 1-F.
Identification and Localization of PTMs on Intact Histone H4
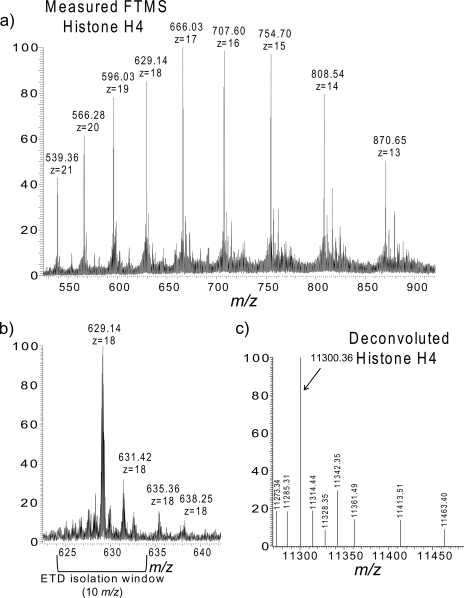
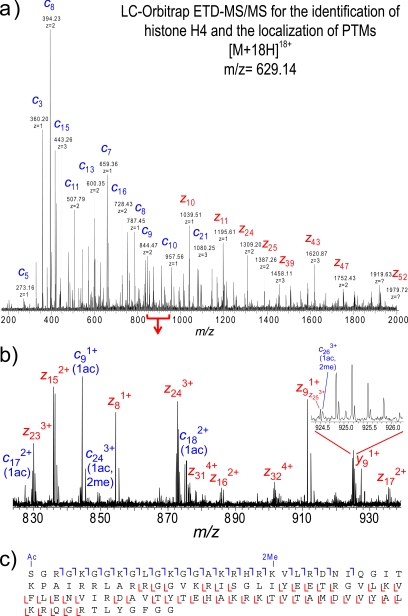
The broadband molecular ion spectrum for histone H4 (m/z 629.14, z = 18) is shown in Fig. 4a. Isotopic resolution was achieved with an Orbitrap resolving power of 60,000 (at m/z 400); there is sufficient resolution to determine the charge state and monoisotopic mass of the intact histones (Fig. 4b). The deconvoluted monoisotopic masses of the most intense species in the molecular ion spectrum are shown in Fig. 4c. The base peak, 11,300.3904, correlates to histone H4 with the loss of a methionine residue and the addition of one acetyl group and two methyl groups (−2.35-ppm mass error; Table I). Using the parameters described under “Experimental Procedures,” histone H4 was the top hit when searching the whole of the mouse database even when searched with the parent mass alone. Confirmation of this identification as well as the localization of the modifications, however, is derived from the fragmentation spectrum.
Fig. 4.
On-line Orbitrap MS of histone H4 molecular ions. a, broadband FT mass spectrum; b, 18+ base peak with ETD isolation window indicated; and c, the deconvoluted histone H4 spectrum. The high resolution mass spectrum acquired in the Orbitrap enables charge state and monoisotopic mass determination necessary for accurate intact protein identification.
Software-assisted and manual data analyses of the ETD fragment ion spectrum acquired in the same run confirmed the identification of histone H4 and putative localization of modifications (Fig. 5). The peak list of the fragment ions generated by Xtract was sufficient to identify histone H4 with N-terminal acetylation and dimethylation at Lys-20 when the data were searched using Protein Prospector. However, further interpretation of the ETD fragment ion spectrum using the FAVA strategy revealed extensive coverage of sequence ion series from both the N and C termini, confirming the protein identification and sites of post-translational modification (Fig. 5c). A total of 129 c and z fragment ions were detected, including at least one fragment ion representing 67% of the possible fragmentation sites in this entire protein sequence (Tables I and II). A mass addition of 42.01 was found on all c ions observed, confirming the localization of the acetylation to the N terminus. The c20 ion corresponding to the cleavage at the Lys-20 residue (and subsequent c ions C-terminal of the Lys-20 residue) was found to be shifted in mass by an additional 28.03 (total of 70.04), indicating a lysine ε-amino dimethylation at this site. No unmethylated or monomethylated c ions were detected C-terminal to Lys-20. In addition to the detection of fragment ions corresponding to the most abundant species isolated, we also detected a c17 ion in the diacetylated state corresponding to N-terminal acetylation and acetylation of Lys-16, a known site of relatively abundant acetylation in mouse embryonic stem cells (14).
Fig. 5.
On-line LC-Orbitrap ETD-MS/MS analysis of intact histone H4 for localization of PTMs. a, ETD fragment ion spectrum; only the most abundant ions are labeled. The spectrum is an average spectrum composite of four MS/MS spectra. b, labeled ETD fragment ions for the mass range of m/z 820–940. c, fragment ion assignments. ETD fragmentation enables the determination of N-terminal loss of methionine and localization of monoacetylation to the N terminus and dimethylation at Lys-20.
Table II. Peak assignments for high resolution ETD MS/MS spectrum of modified histone H4 as shown in Fig. 5.
For some higher mass peaks, the monoisotopic peak was not observed; therefore, higher isotopic peaks were used.
| Ion type | Ion no. | PTM(s) | Charge | m/z measured | MH+ measured | MH+ calculated | Error |
|---|---|---|---|---|---|---|---|
| Da | Da | ppm | |||||
| c | 3 | Acetyl | 1 | 360.199 | 360.199 | 360.199 | −1.11 |
| c | 4 | Acetyl | 1 | 417.220 | 417.220 | 417.221 | −1.44 |
| c | 5 | Acetyl | 1 | 545.315 | 545.315 | 545.315 | −0.92 |
| c | 5 | Acetyl | 2 | 273.161 | 545.315 | 545.315 | −0.86 |
| c | 6 | Acetyl | 1 | 602.336 | 602.336 | 602.337 | −1.00 |
| c | 6 | Acetyl | 2 | 301.672 | 602.336 | 602.337 | −1.61 |
| c | 7 | Acetyl | 1 | 659.358 | 659.358 | 659.358 | −0.76 |
| c | 7 | Acetyl | 2 | 330.183 | 659.358 | 659.358 | −0.86 |
| c | 8 | Acetyl | 1 | 787.453 | 787.453 | 787.453 | −0.51 |
| c | 8 | Acetyl | 2 | 394.230 | 787.452 | 787.453 | −1.23 |
| c | 9 | Acetyl | 1 | 844.474 | 844.474 | 844.475 | −0.71 |
| c | 9 | Acetyl | 2 | 422.740 | 844.474 | 844.475 | −1.50 |
| c | 9 | Acetyl | 3 | 282.163 | 844.474 | 844.475 | −1.11 |
| c | 10 | Acetyl | 1 | 957.558 | 957.558 | 957.559 | −0.52 |
| c | 10 | Acetyl | 2 | 479.282 | 957.557 | 957.559 | −1.74 |
| c | 11 | Acetyl | 1 | 1014.580 | 1014.580 | 1014.580 | −0.10 |
| c | 11 | Acetyl | 2 | 507.793 | 1014.579 | 1014.580 | −1.74 |
| c | 11 | Acetyl | 3 | 338.865 | 1014.580 | 1014.580 | −0.14 |
| c | 12 | Acetyl | 1 | 1142.676 | 1142.676 | 1142.675 | 0.18 |
| c | 12 | Acetyl | 2 | 571.841 | 1142.674 | 1142.675 | −1.20 |
| c | 12 | Acetyl | 3 | 381.563 | 1142.674 | 1142.675 | −1.00 |
| c | 13 | Acetyl | 1 | 1199.696 | 1199.696 | 1199.697 | −0.42 |
| c | 13 | Acetyl | 2 | 600.352 | 1199.696 | 1199.697 | −0.81 |
| c | 13 | Acetyl | 3 | 400.570 | 1199.695 | 1199.697 | −1.28 |
| c | 14 | Acetyl | 1 | 1256.719 | 1256.719 | 1256.718 | 0.80 |
| c | 14 | Acetyl | 2 | 628.862 | 1256.717 | 1256.718 | −1.01 |
| c | 14 | Acetyl | 3 | 419.577 | 1256.716 | 1256.718 | −1.38 |
| c | 15 | Acetyl | 1 | 1327.756 | 1327.756 | 1327.755 | 0.15 |
| c | 15 | Acetyl | 2 | 664.381 | 1327.754 | 1327.755 | −0.73 |
| c | 15 | Acetyl | 3 | 443.256 | 1327.753 | 1327.755 | −1.39 |
| c | 16 | Acetyl | 2 | 728.428 | 1455.848 | 1455.850 | −1.35 |
| c | 16 | Acetyl | 3 | 485.954 | 1455.848 | 1455.850 | −1.54 |
| c | 17 | 2 acetyl | 2 | 827.484 | 1653.961 | 1653.962 | −0.47 |
| c | 17 | Acetyl | 2 | 806.480 | 1611.952 | 1611.951 | 0.58 |
| c | 17 | Acetyl | 3 | 537.988 | 1611.948 | 1611.951 | −2.13 |
| c | 17 | Acetyl | 4 | 403.742 | 1611.945 | 1611.951 | −3.98 |
| c | 18 | Acetyl | 2 | 875.008 | 1749.009 | 1749.010 | −0.78 |
| c | 18 | Acetyl | 3 | 583.674 | 1749.009 | 1749.010 | −0.94 |
| c | 18 | Acetyl | 4 | 438.007 | 1749.005 | 1749.010 | −3.04 |
| c | 19 | Acetyl | 2 | 953.059 | 1905.111 | 1905.111 | −0.35 |
| c | 19 | Acetyl | 3 | 635.708 | 1905.109 | 1905.111 | −1.49 |
| c | 19 | Acetyl | 4 | 477.032 | 1905.107 | 1905.111 | −2.31 |
| c | 21 | Acetyl + dimethyl | 2 | 1080.657 | 2160.306 | 2160.306 | 0.01 |
| c | 22 | Acetyl + dimethyl | 3 | 758.467 | 2273.386 | 2273.390 | −2.00 |
| c | 23 | Acetyl + dimethyl | 3 | 810.500 | 2429.486 | 2429.491 | −2.16 |
| c | 23 | Acetyl + dimethyl | 4 | 608.128 | 2429.491 | 2429.491 | 0.04 |
| c | 24 | Acetyl + dimethyl | 3 | 848.846 | 2544.524 | 2544.518 | 2.42 |
| c | 26 | Acetyl + dimethyl | 3 | 924.552 | 2771.642 | 2771.645 | −1.19 |
| z | 8 | 1 | 854.428 | 854.428 | 854.428 | −0.47 | |
| z | 9 | 1 | 911.449 | 911.449 | 911.450 | −0.33 | |
| z | 10 | 1 | 1039.508 | 1039.508 | 1039.508 | −0.29 | |
| z | 11 | 1 | 1195.609 | 1195.609 | 1195.609 | 0.08 | |
| z | 11 | 2 | 598.308 | 1195.608 | 1195.609 | −1.15 | |
| z | 12 | 1 | 1323.709 | 1323.709 | 1323.704 | 3.32 | |
| z | 12 | 2 | 662.356 | 1323.704 | 1323.704 | −0.36 | |
| z | 13 | 1 | 1437.796 | 1437.796 | 1437.796 | 0.21 | |
| z | 13 | 2 | 718.897 | 1436.787 | 1436.788 | −1.09 | |
| Da | Da | ppm | |||||
| z | 14 | 1 | 1508.832 | 1508.832 | 1508.833 | −0.93 | |
| z | 14 | 2 | 754.416 | 1507.824 | 1507.825 | −0.97 | |
| z | 15 | 1 | 1670.894 | 1670.894 | 1670.889 | 3.17 | |
| z | 15 | 2 | 835.948 | 1670.888 | 1670.889 | −0.58 | |
| z | 16 | 1 | 1769.973 | 1769.973 | 1769.957 | 9.04 | |
| z | 16 | 2 | 885.482 | 1769.957 | 1769.957 | 0.02 | |
| z | 17 | 2 | 935.019 | 1869.030 | 1869.026 | 2.32 | |
| z | 17 | 3 | 623.679 | 1869.023 | 1869.026 | −1.20 | |
| z | 18 | 2 | 992.535 | 1984.062 | 1984.053 | 4.65 | |
| z | 19 | 2 | 1058.050 | 2115.092 | 2115.093 | −0.41 | |
| z | 19 | 3 | 705.702 | 2115.091 | 2115.093 | −0.87 | |
| z | 20 | 2 | 1093.565 | 2186.124 | 2186.130 | −3.01 | |
| z | 20 | 3 | 729.381 | 2186.129 | 2186.130 | −0.61 | |
| z | 21 | 2 | 1144.092 | 2287.176 | 2287.178 | −0.73 | |
| z | 21 | 3 | 763.064 | 2287.177 | 2287.178 | −0.41 | |
| z | 22 | 2 | 1193.616 | 2386.225 | 2386.246 | −9.00 | |
| z | 22 | 3 | 796.087 | 2386.245 | 2386.246 | −0.39 | |
| z | 23 | 2 | 1244.166 | 2487.324 | 2487.294 | 12.15 | |
| z | 23 | 3 | 829.769 | 2487.292 | 2487.294 | −0.82 | |
| z | 24 | 2 | 1308.199 | 2615.391 | 2615.389 | 0.74 | |
| z | 24 | 3 | 872.467 | 2615.386 | 2615.389 | −1.12 | |
| z | 25 | 2 | 1386.752 | 2772.496 | 2772.498 | −0.71 | |
| z | 25 | 3 | 924.502 | 2771.490 | 2771.490 | 0.13 | |
| z | 26 | 2 | 1450.793 | 2900.580 | 2900.593 | −4.54 | |
| z | 26 | 3 | 967.198 | 2899.579 | 2899.585 | −1.88 | |
| z | 26 | 4 | 725.657 | 2899.608 | 2899.585 | 7.89 | |
| z | 27 | 2 | 1486.324 | 2971.641 | 2971.630 | 3.61 | |
| z | 27 | 3 | 991.214 | 2971.628 | 2971.630 | −0.55 | |
| z | 28 | 2 | 1554.848 | 3108.689 | 3108.689 | 0.20 | |
| z | 28 | 3 | 1036.566 | 3107.682 | 3107.681 | 0.44 | |
| z | 28 | 4 | 777.674 | 3107.674 | 3107.681 | −2.11 | |
| z | 29 | 2 | 1619.371 | 3237.734 | 3237.731 | 0.87 | |
| z | 29 | 3 | 1079.914 | 3237.726 | 3237.731 | −1.65 | |
| z | 29 | 4 | 809.937 | 3236.726 | 3236.724 | 0.83 | |
| z | 30 | 3 | 1113.603 | 3338.793 | 3338.779 | 4.27 | |
| z | 31 | 2 | 1751.432 | 3501.856 | 3501.842 | 3.89 | |
| z | 31 | 3 | 1167.617 | 3500.835 | 3500.835 | 0.13 | |
| z | 31 | 4 | 875.970 | 3500.857 | 3500.835 | 6.31 | |
| z | 32 | 2 | 1801.951 | 3602.894 | 3602.890 | 1.15 | |
| z | 32 | 3 | 1201.298 | 3601.878 | 3601.882 | −1.18 | |
| z | 32 | 4 | 901.222 | 3601.864 | 3601.882 | −5.00 | |
| z | 34 | 3 | 1258.339 | 3773.002 | 3772.996 | 1.69 | |
| z | 34 | 4 | 943.753 | 3771.989 | 3771.988 | 0.24 | |
| z | 35 | 2 | 1944.512 | 3888.016 | 3888.023 | −1.64 | |
| z | 35 | 3 | 1296.682 | 3888.032 | 3888.023 | 2.38 | |
| z | 35 | 4 | 972.505 | 3886.999 | 3887.015 | −3.94 | |
| z | 38 | 3 | 1419.430 | 4256.276 | 4256.276 | −0.08 | |
| z | 39 | 3 | 1457.453 | 4370.344 | 4370.319 | 5.64 | |
| z | 39 | 4 | 1093.334 | 4370.312 | 4370.319 | −1.60 | |
| z | 40 | 3 | 1500.466 | 4499.384 | 4499.362 | 4.93 | |
| z | 41 | 3 | 1538.157 | 4612.458 | 4612.446 | 2.59 | |
| z | 42 | 3 | 1587.176 | 4759.512 | 4759.514 | −0.39 | |
| z | 42 | 4 | 1190.382 | 4758.505 | 4758.506 | −0.19 | |
| z | 43 | 3 | 1619.871 | 4857.599 | 4857.575 | 5.01 | |
| z | 43 | 4 | 1215.402 | 4858.587 | 4858.583 | 1.01 | |
| z | 44 | 3 | 1663.225 | 4987.660 | 4987.685 | −5.02 | |
| z | 44 | 4 | 1247.428 | 4986.691 | 4986.677 | 2.65 | |
| z | 45 | 3 | 1700.923 | 5100.756 | 5100.769 | −2.67 | |
| z | 48 | 3 | 1804.996 | 5412.974 | 5412.960 | 2.60 | |
| Da | Da | ppm | |||||
| z | 48 | 4 | 1353.999 | 5412.975 | 5412.960 | 2.79 | |
| z | 49 | 3 | 1838.663 | 5513.973 | 5514.008 | −6.30 | |
| z | 50 | 3 | 1882.023 | 5644.054 | 5644.058 | −0.75 | |
| z | 50 | 4 | 1411.767 | 5644.046 | 5644.058 | −2.16 | |
| z | 51 | 3 | 1925.030 | 5773.075 | 5773.101 | −4.58 | |
| z | 51 | 4 | 1443.769 | 5772.053 | 5772.093 | −6.90 | |
| z | 52 | 3 | 1979.384 | 5936.137 | 5936.164 | −4.52 | |
| z | 56 | 4 | 1577.609 | 6307.416 | 6307.394 | 3.50 | |
| z | 57 | 4 | 1605.875 | 6420.479 | 6420.478 | 0.19 | |
| z | 58 | 4 | 1644.648 | 6575.571 | 6575.571 | −0.08 | |
| z | 61 | 4 | 1715.690 | 6859.738 | 6859.756 | −2.64 | |
| z | 62 | 4 | 1730.205 | 6917.799 | 6917.785 | 1.94 | |
| z | 63 | 4 | 1769.228 | 7073.888 | 7073.886 | 0.27 | |
| y | 9 | 1 | 927.468 | 927.468 | 927.468 | −0.32 | |
| y | 11 | 1 | 1211.627 | 1211.627 | 1211.628 | −0.83 | |
| y | 11 | 2 | 606.317 | 1211.626 | 1211.628 | −1.54 | |
| y | 12 | 2 | 670.365 | 1339.722 | 1339.723 | −0.87 | |
| y | 14 | 1 | 1524.853 | 1524.853 | 1524.852 | 0.72 | |
| y | 14 | 2 | 762.425 | 1523.843 | 1523.844 | −0.90 | |
| y | 31 | 3 | 1172.960 | 3516.865 | 3516.853 | 3.29 | |
| Unassigned | 2 | 622.864 | 1244.721 | ||||
| Unassigned | 2 | 499.310 | 997.612 | ||||
| Unassigned | 1 | 997.612 | 997.612 | ||||
| Unassigned | 2 | 563.339 | 1125.671 | ||||
| Unassigned | 1 | 527.821 | 527.821 | ||||
| Unassigned | 2 | 591.851 | 1182.694 | ||||
| Unassigned | 2 | 627.388 | 1253.769 | ||||
| Unassigned | 2 | 773.967 | 1546.926 | ||||
| Unassigned | 2 | 798.469 | 1595.931 | ||||
| Unassigned | 2 | 802.982 | 1604.956 | ||||
| Unassigned | 2 | 805.161 | 1609.314 | ||||
| Unassigned | 2 | 867.501 | 1733.994 | ||||
| Unassigned | 3 | 878.141 | 2632.410 | ||||
| Unassigned | 2 | 1563.359 | 3125.710 |
Detection and Relative Quantitation of Modified Histone H4 Peptides following Asp-N Digestion
Digestion of unfractionated histone extracts with the endoproteinase Asp-N resulted in the generation of a large number of peptides, including a 23-amino acid peptide corresponding to the histone H4 N-terminal tail. This region of histone H4 in murine embryonic stem cells is known to be the most heavily modified and enables combinatorial analysis of the most common histone H4 modifications: acetylation at the N terminus, Lys-5, Lys-8, Lys-12, and Lys-16 as well as mono-, di-, and trimethylation at Lys-20 (14).
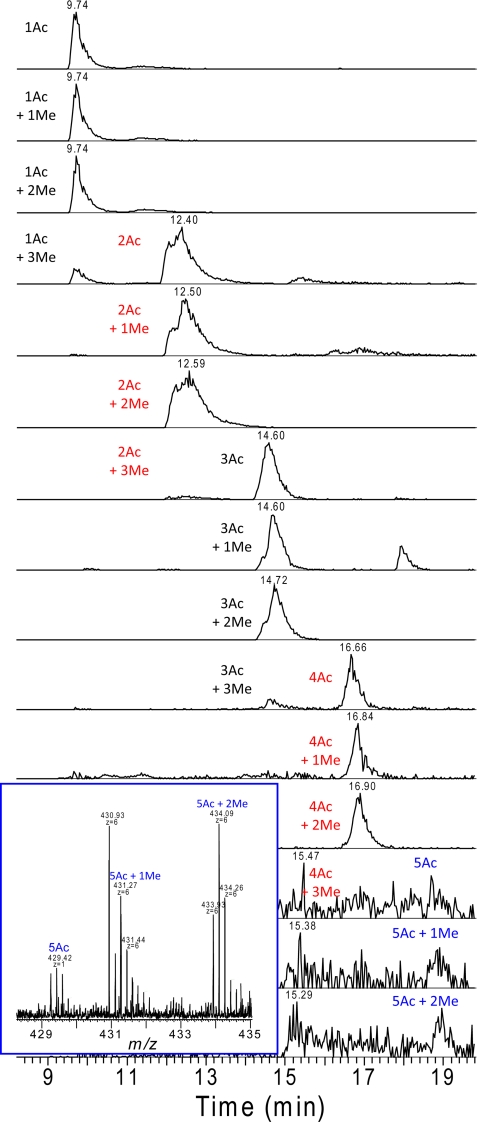
When analyzed by on-line RP UPLC MS, the histone H4 N-terminal 23-mers modified with each combination of one to five acetylations and zero to three methylations were detected. These peptides are chromatographically separated according to their acetylation status as shown in Fig. 6. Thus, the isobaric trimethylated and acetylated peptides are completely resolved, enabling relative quantitation by comparing peak intensities (Table III). The most abundant species is the dimethylated species with one acetylation. As the number of acetylations increased, the relative abundance of the peptides decreased. Although the extracted ion chromatogram has low signal to noise at five acetylations, the unmodified, mono-, and dimethylated versions can clearly be detected (Fig. 6, inset). At all levels of acetylation, the dimethylated species was the most abundant species, the unmethylated and monomethylated species were nearly equivalent in intensity to each other, and the trimethylated species was of lower abundance.
Fig. 6.
Extracted ion chromatograms showing typical separation of differentially modified histone H4 N-terminal tails according to level of acetylation. An increased level of acetylation increased the peptide retention time of the peptides; however, the level of methylation did not affect the retention time. Although signal to noise is high for the extracted ion chromatograms of peptides with five acetylations, the inset shows the sufficient ion intensity in the MS spectrum.
Table III. Relative abundance of histone H4 N-terminal tail peptides with differing levels of post-translational modification.
| PTMs | Relative abundance ± S.D. |
|---|---|
| 1Ac | 8.17 ± 4.05 |
| 1Ac + 1Me | 8.52 ± 2.48 |
| 1Ac + 2Me | 34.85 ± 3.86 |
| 1Ac + 3Me | 1.67 ± 0.03 |
| 2Ac | 5.46 ± 0.82 |
| 2Ac + 1Me | 3.51 ± 0.15 |
| 2Ac + 2Me | 11.97 ± 0.86 |
| 2Ac + 3Me | 1.20 ± 0.28 |
| 3Ac | 7.91 ± 3.72 |
| 3Ac + 1Me | 3.09 ± 1.21 |
| 3Ac + 2Me | 10.31 ± 4.05 |
| 3Ac + 3Me | 0.39 ± 0.23 |
| 4Ac | 1.38 ± 0.34 |
| 4Ac + 1Me | 0.42 ± 0.05 |
| 4Ac + 2Me | 1.83 ± 0.47 |
| 4Ac + 3Me | 0.11 ± 0.07 |
| 5Ac | 0.04 ± 0.03 |
| 5Ac + 1Me | 0.14 ± 0.07 |
| 5Ac + 2Me | 0.14 ± 0.08 |
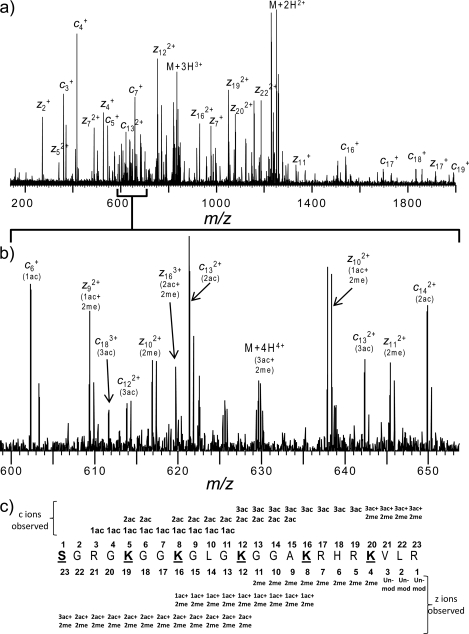
The identities of the peptides were confirmed from analysis of the electrospray ETD spectra. A representative high resolution ETD mass spectrum for the histone H4 N-terminal tail [M + 6H]6+ is shown in Fig. 7. This particular species contains an additional three acetyl and two methyl moieties. Use of the FAVA strategy (25) permitted detection and assignment of a complete c and z fragment ion series. All members of the c ion series were detected bearing at least one acetyl moiety (42.01), indicating the acetylation of all N termini and thus indicating that the remaining two acetyl and two methyl groups were internal peptide modifications. Both methyl residues were localized to Lys-20 based upon the observation that the z1–z3 fragment ions were unmodified and all z4 ions had a mass shift of 28.31 Da, the mass of two methyl residues. Acetylation, although not stoichiometric acetylation, was detected at Lys-16 and Lys-12 as indicated by the presence of both the acetylated, dimethylated z8 ion and the dimethylated z8 ion as well as the acetylated, dimethylated z12 ion and the diacetylated, dimethylated z12 ion, respectively. Lys-8 acetylation was indicated based upon the fact that all ions N-terminal of z16 were completely modified with two acetyl and two methyl moieties. In addition, the c ion series was used to confirm the presence of Lys-5 acetylation as both monoacetylated and diacetylated c5 ions were detected indicating substoichiometric Lys-5 acetylation.
Fig. 7.
LC-ETD MS/MS analysis of modified histone H4 N-terminal peptides following unfractionated histone Asp-N digestion. a, upper panel, high resolution ETD fragment ion spectrum of the histone H4 N-terminal tail with the addition of three acetylations and two methylations; lower panel, m/z range of 600–650. b, detected fragment ion assignments with variable modifications indicated.
DISCUSSION
Here we present the data obtained from on-line separation, detection, and identification of intact, unfractionated histones. The core histones of mouse embryonic stem cells range from 11 to 15 kDa in size, all with relatively basic N-terminal tails. The effectiveness of RP UPLC with on-line ETD for carrying out analysis of intact histones has been presented using ∼45 pmol of histones. Sufficient separation of the core histones enabled multiple ETD fragmentations over the elution of each protein, requiring ∼8 s per scan. With the ETD fragmentation implemented on an Orbitrap mass analyzer (28), we were able to record high resolution data for both the molecular ions and the fragment ions that are necessary for the experiments carried out at the intact level. This study represents the first analysis of an intact bulk histone mixture taking advantage of the detailed sequence ion information provided by ETD mass analysis at high resolution. Although the dynamic range proves to be a limitation, sufficient fragmentation was obtained to identify the major species, including isoform determination and post-translational modification assignment.
Only recently have new technological advances based on ECD and ETD energy deposition processes begun to permit these studies. Gaining the ability to characterize multiple post-translational modifications on intact histone molecules provides the rigorous modification occupancy required to decipher biological functions. This was shown by early ECD work using FTICRMS on the post-translational occupancies of histone variants H2B.1 and H2B.2 isolated from Tetrahymena (12). MS/MS analysis of intact histones has been performed by direct infusion following off-line fractionation for histone H4 (13), histone H2B (29), histone H2A (30), and (to a lesser extent because of the complexity of the PTMs) histone H3 (31). Young et al. (32) have recently reported the separation and detection of large histone H3 peptides according to both their acetylation and methylation status by on-line ETD MS utilizing a “saltless” pH gradient weak cation exchange-hydrophilic interaction liquid chromatography. Wu et al. (33) have integrated the analysis of intact proteins and enzymatic digests to identify proteins, modifications, and genetic variants by utilizing high resolution RP LC intact protein separations coupled on line with an FTICR mass spectrometer to profile and tentatively identify modified proteins in conjunction with bottom-up analysis of the corresponding LC fractions.
The data processing work flow for protein identification, protein isoform determination, and PTM localization, as indicated in Fig. 1, included analyses of both the on-line MS and the on-line ETD spectra. Preliminary deconvoluted, monoisotopic MH+ masses of the molecular and fragment ions were obtained using the Xtract software (Thermo Scientific) on both the broadband MS and the ETD fragment ion spectra. These preliminary mass values were submitted to the Protein Prospector MS-TAG search algorithm (an in-house software), and putative protein isoform and putative sites of PTM were obtained for the major positional isomer present. As a variety of protein isoforms/positional modification variants are possible within a few ppm mass cutoff, further more thorough analysis of the actual fragment ion spectra is essential. We have experienced three inherent drawbacks in using peak extraction software for complex spectrum analysis. These include the fact that this software often (a) assigns incorrect MH+ values, (b) fails to find low abundance ions, and (c) fails to deconvolute overlapping isotopic clusters. For these reasons, comprehensive fragment ion assignment was obtained for each ETD spectrum with the assistance of FAVA (25). These assignments establish the protein isoforms and the actual sites of PTM attachments. The FAVA strategy permits searches of raw data files for fragment ions of a particular input sequence, which may or may not include site-specific modifications, that resulted in sensitive and accurate assignment of histone H4 fragment ions.
With this data processing work flow, we were able to identify eight different histone isoforms during the 15-min elution (Table I). The advantages of on-line ETD were exemplified when the molecular weight of one histone was equivalent to three isobaric histone H2B isoforms. The ETD fragmentation enabled the determination of histone H2B type 1-F as the only H2B isoform present in the spectrum. In addition, the ETD fragmentation was essential in localizing PTMs. For the majority of the histones, the most abundant species detected were present as unmodified or only modified by acetylation at the N termini. The most abundant histone H4 species, however, had N-terminal acetylation and dimethylation at Lys-20. We also detected a further acetylated c17 ion in the ETD spectrum. The minimum isolation window for the intact ETD experiments was 10 m/z. This isolation window encompassed more than a single precursor ion species, and this further acetylated species was also included, albeit at a lower abundance (Fig. 4), which upon fragmentation “contaminated” the fragment ion spectrum of the isolated species intended.
In addition to the limitations that the 10 m/z isolation window presents when dealing with co-eluting intact proteins (namely, modified versions of the same protein), another limitation is the low dynamic range for the detection of low abundance modified species. Analysis of Asp-N digests of the same unfractionated acid-extracted histones led to the detection of a plethora of modified histone H4 N-terminal tails (Figs. 6 and 7 and Table III), which were previously undetected in the intact histone analysis. Asp-N digestion of histone H4 generates a 23-residue N-terminal peptide that permits combinatorial analysis of the most common histone H4 modifications in mouse embryonic stem cells: acetylation at the N terminus, Lys-5, Lys-8, Lys-12, and Lys-16 as well as mono-, di-, and trimethylation at Lys-20.
With a possible five acetyl and three methyl groups present, there are 15 possible N-terminal histone H4 peptides with variable levels of modification. The percentage of each species present is listed in Table III, which indicates the two most abundant species as the monoacetylated, dimethylated species and the diacetylated, dimethylated species with ∼34 and 12% of total ion abundance, respectively. This is consistent with the intact protein analysis (Fig. 4); it illustrates the low dynamic range of the intact analysis as the less abundant modified species were not detected in the analysis conducted at the intact level.
In addition to the analysis of intact histones, the work flow used here will likely be applicable to the on-line analysis of a variety of intact protein families or intact protein subpopulations. The ability to perform fragmentation with an on-line LC time scale diminishes the need for complete purification of a single protein species that is often required for analysis by direct infusion experiments. In effect, sample handling and loss are reduced, and the on-line experiment requires less sample compared with the direct infusion experiment, thus making intact MS/MS analysis more accessible for difficult to purify and/or low abundance proteins. Although near complete sequence information can be obtained for small proteins, enabling the localization of post-translational modifications, this may not be the case for larger proteins. With that in mind, however, essential information can still be obtained regarding protein identification and the specific presence of modifications and proteolytic processing of the N and C termini.
CONCLUSIONS
We have described an on-line RP UPLC Orbitrap-ETD approach for the profiling of histone extracts for isoform and PTM variants. This approach combined the speed, sensitivity, and high resolution capabilities of the LTQ-Orbitrap with the efficiency of ETD fragmentation to characterize the genetic variants and PTMs of the most abundant species in bulk histone extracts of mouse embryonic stem cells in a single LC ETD run. However, because of the relatively wide isolation window required for intact protein fragmentation and the low dynamic range, further advances will be required to improve the on-line separation of modified intact histones and the technical methods by which the ions are isolated for ETD fragmentation. Although data were only presented for the analysis of intact histones, we suggest that this method will be directly applicable to the comprehensive analysis of proteins of similar size for identification and site-specific localization of modifications. In addition, protein identification experiments for larger proteins are likely, although localization of modifications not restricted to the N and C termini may still remain a challenge.
Acknowledgments
We thank Shenheng Guan for valuable discussions.
* This work was supported, in whole or in part, by National Institutes of Health Grant NCRR P41RR001614 from the Biomedical Research Technology Program of the National Center for Research Resources through the Bio-Organic Biomedical Mass Spectrometry Resource at the University of California, San Francisco (A. L. Burlingame, Director).
1 The abbreviations used are:
- PTM
- post-translational modification
- ECD
- electron capture dissociation
- ETD
- electron transfer dissociation
- FAVA
- Fragment Assignment by Visual Assistance
- RP
- reversed phase
- UPLC
- ultraperformance LC.
REFERENCES
- 1.Burlingame A. L. (ed) (2005) Methods in Enzymology, Vol. 402, Elsevier Academic Press, San Diego, CA [Google Scholar]
- 2.Gstaiger M., Aebersold R. (2009) Applying mass spectrometry-based proteomics to genetics, genomics and network biology. Nat. Rev. Genet 10, 617–627 [DOI] [PubMed] [Google Scholar]
- 3.Cravatt B. F., Simon G. M., Yates J. R., 3rd (2007) The biological impact of mass-spectrometry-based proteomics. Nature 450, 991–1000 [DOI] [PubMed] [Google Scholar]
- 4.Yates J. R., Ruse C. I., Nakorchevsky A. (2009) Proteomics by mass spectrometry: approaches, advances, and applications. Annu. Rev. Biomed. Eng 11, 49–79 [DOI] [PubMed] [Google Scholar]
- 5.Zubarev R. A., Kelleher N. L., McLafferty F. W. (1998) Electron capture dissociation of multiply charged protein cations. A nonergodic process. J. Am. Chem. Soc 120, 3265–3266 [Google Scholar]
- 6.Syka J. E., Coon J. J., Schroeder M. J., Shabanowitz J., Hunt D. F. (2004) Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U.S.A 101, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zubarev R. A. (2004) Electron-capture dissociation tandem mass spectrometry. Curr. Opin. Biotechnol 15, 12–16 [DOI] [PubMed] [Google Scholar]
- 8.Cooper H. J., Håkansson K., Marshall A. G. (2005) The role of electron capture dissociation in biomolecular analysis. Mass Spectrom. Rev 24, 201–222 [DOI] [PubMed] [Google Scholar]
- 9.McAlister G. C., Phanstiel D., Good D. M., Berggren W. T., Coon J. J. (2007) Implementation of electron-transfer dissociation on a hybrid linear ion trap-orbitrap mass spectrometer. Anal. Chem 79, 3525–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalkley R. J., Thalhammer A., Schoepfer R., Burlingame A. L. (2009) Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc. Natl. Acad. Sci. U.S.A 106, 8894–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikesh L. M., Ueberheide B., Chi A., Coon J. J., Syka J. E., Shabanowitz J., Hunt D. F. (2006) The utility of ETD mass spectrometry in proteomic analysis. Biochim. Biophys. Acta 1764, 1811–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medzihradszky K. F., Zhang X., Chalkley R. J., Guan S., McFarland M. A., Chalmers M. J., Marshall A. G., Diaz R. L., Allis C. D., Burlingame A. L. (2004) Characterization of Tetrahymena histone H2B variants and posttranslational populations by electron capture dissociation (ECD) Fourier transform ion cyclotron mass spectrometry (FT-ICR MS). Mol. Cell. Proteomics 3, 872–886 [DOI] [PubMed] [Google Scholar]
- 13.Pesavento J. J., Bullock C. R., LeDuc R. D., Mizzen C. A., Kelleher N. L. (2008) Combinatorial modification of human histone H4 quantitated by two-dimensional liquid chromatography coupled with top down mass spectrometry. J. Biol. Chem 283, 14927–14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phanstiel D., Brumbaugh J., Berggren W. T., Conard K., Feng X., Levenstein M. E., McAlister G. C., Thomson J. A., Coon J. J. (2008) Mass spectrometry identifies and quantifies 74 unique histone H4 isoforms in differentiating human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A 105, 4093–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burlingame A. L., Zhang X., Chalkley R. J. (2005) Mass spectrometric analysis of histone posttranslational modifications. Methods 36, 383–394 [DOI] [PubMed] [Google Scholar]
- 16.Garcia B. A., Shabanowitz J., Hunt D. F. (2007) Characterization of histones and their post-translational modifications by mass spectrometry. Curr. Opin. Chem. Biol 11, 66–73 [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove M. S. (2007) Histone proteomics and the epigenetic regulation of nucleosome mobility. Expert Rev. Proteomics 4, 465–478 [DOI] [PubMed] [Google Scholar]
- 18.Ruthenburg A. J., Li H., Patel D. J., Allis C. D. (2007) Multivalent engagement of chromatin modifications by linked binding modules. Nat. Rev. Mol. Cell Biol 8, 983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson C. L., Laniel M. A. (2004) Histones and histone modifications. Curr. Biol 14, R546–R551 [DOI] [PubMed] [Google Scholar]
- 20.Freitas M. A., Sklenar A. R., Parthun M. R. (2004) Application of mass spectrometry to the identification and quantification of histone post-translational modifications. J. Cell. Biochem 92, 691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taverna S. D., Li H., Ruthenburg A. J., Allis C. D., Patel D. J. (2007) How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat. Struct. Mol. Biol 14, 1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraga M. F., Esteller M. (2005) Towards the human cancer epigenome: a first draft of histone modifications. Cell Cycle 4, 1377–1381 [DOI] [PubMed] [Google Scholar]
- 23.Couture J. F., Collazo E., Ortiz-Tello P. A., Brunzelle J. S., Trievel R. C. (2007) Specificity and mechanism of JMJD2A, a trimethyllysine-specific histone demethylase. Nat. Struct. Mol. Biol 14, 689–695 [DOI] [PubMed] [Google Scholar]
- 24.Hake S. B., Garcia B. A., Kauer M., Baker S. P., Shabanowitz J., Hunt D. F., Allis C. D. (2005) Serine 31 phosphorylation of histone variant H3.3 is specific to regions bordering centromeres in metaphase chromosomes. Proc. Natl. Acad. Sci. U.S.A 102, 6344–6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan S., Burlingame A. L. (November25, 2009) Data processing algorithms for analysis of high resolution MSMS spectra of peptides with complex patterns of posttranslational modifications. Mol. Cell. Proteomics 10.1074/mcp.M900431-MCP200, 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalkley R. J., Baker P. R., Medzihradszky K. F., Lynn A. J., Burlingame A. L. (2008) In-depth analysis of tandem mass spectrometry data from disparate instrument types. Mol. Cell. Proteomics 7, 2386–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn D. M., Zubarev R. A., McLafferty F. W. (2000) Automated reduction and interpretation of high resolution electrospray mass spectra of large molecules. J. Am. Soc. Mass Spectrom 11, 320–332 [DOI] [PubMed] [Google Scholar]
- 28.McAlister G. C., Berggren W. T., Griep-Raming J., Horning S., Makarov A., Phanstiel D., Stafford G., Swaney D. L., Syka J. E., Zabrouskov V., Coon J. J. (2008) A proteomics grade electron transfer dissociation-enabled hybrid linear ion trap-orbitrap mass spectrometer. J. Proteome Res 7, 3127–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siuti N., Roth M. J., Mizzen C. A., Kelleher N. L., Pesavento J. J. (2006) Gene-specific characterization of human histone H2B by electron capture dissociation. J. Proteome Res 5, 233–239 [DOI] [PubMed] [Google Scholar]
- 30.Boyne M. T., 2nd, Pesavento J. J., Mizzen C. A., Kelleher N. L. (2006) Precise characterization of human histones in the H2A gene family by top down mass spectrometry. J. Proteome Res 5, 248–253 [DOI] [PubMed] [Google Scholar]
- 31.Thomas C. E., Kelleher N. L., Mizzen C. A. (2006) Mass spectrometric characterization of human histone H3: a bird's eye view. J. Proteome Res 5, 240–247 [DOI] [PubMed] [Google Scholar]
- 32.Young N. L., DiMaggio P. A., Plazas-Mayorca M. D., Baliban R. C., Floudas C. A., Garcia B. A. (2009) High throughput characterization of combinatorial histone codes. Mol. Cell. Proteomics 8, 2266–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S., Lourette N. M., Toliæ N., Zhao R., Robinson E. W., Tolmachev A. V., Smith R. D., Pasa-Toliæ L. (2009) An integrated top-down and bottom-up strategy for broadly characterizing protein isoforms and modifications. J. Proteome Res 8, 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]