Abstract
Loss-of-function DJ-1 (PARK7) mutations have been linked with a familial form of early onset Parkinson disease. Numerous studies have supported the role of DJ-1 in neuronal survival and function. Our initial studies using DJ-1-deficient neurons indicated that DJ-1 specifically protects the neurons against the damage induced by oxidative injury in multiple neuronal types and degenerative experimental paradigms, both in vitro and in vivo. However, the manner by which oxidative stress-induced death is ameliorated by DJ-1 is not completely clear. We now present data that show the involvement of DJ-1 in modulation of AKT, a major neuronal prosurvival pathway induced upon oxidative stress. We provide evidence that DJ-1 promotes AKT phosphorylation in response to oxidative stress induced by H2O2 in vitro and in vivo following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment. Moreover, we show that DJ-1 is necessary for normal AKT-mediated protective effects, which can be bypassed by expression of a constitutively active form of AKT. Taken together, these data suggest that DJ-1 is crucial for full activation of AKT upon oxidative injury, which serves as one explanation for the protective effects of DJ-1.
Keywords: neurodegeneration, Parkinson disease, reactive oxygen species
Individuals with homozygous loss-of-function mutations of DJ-1 (PARK7) have been clinically characterized with familial early onset Parkinson disease (PD) (1, 2). Although the physiological role of DJ-1 is not completely understood, several lines of evidence indicate a protective role for DJ-1 in multiple models of neuronal and nonneuronal oxidative stress-induced cell death (3–7). For example, we have previously shown that genetic ablation of DJ-1 in mice hyper-sensitizes dopamine neurons to the toxic effects induced by the mitochondrial toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). This sensitivity was reversed by the induction of virally delivered human DJ-1 (8). These observations are in line with data by other groups showing sensitivity of dopaminergic neurons in DJ-1–deficient Drosophila models, as well as increased susceptibility to oxidative stress in vitro (9–11). To further support the importance of DJ-1 in managing oxidative stress, we provided evidence showing that DJ-1 protects the brain against ischemic injury that models clinical stroke. Moreover, our data indicated a direct correlation between DJ-1 neuroprotective activity and the reduced levels of oxidized DNA nucleotide species, 8-oxo guanine, a marker of oxidative damage (12).
Despite the fact that the neuroprotective role of DJ-1 has been consistently shown in multiple models of neurodegeneration, the exact mechanism of the neuroprotective function has not been fully elucidated. A direct antioxidant property of DJ-1 as a reactive oxygen species (ROS) scavenger has been proposed as a mechanism to overcome oxidative stress (7, 13). In fact, recombinant human DJ-1 confers some ROS scavenging activity; however, this activity is much weaker than any known peroxidase, thus not fully explaining its neuroprotective function (10, 13). Several alternative mechanisms to account for the neuroprotective function of DJ-1 have been suggested. For example, via its putative role in transcription regulation (14), DJ-1 up-regulates the expression of other antioxidant genes, such as glutathione synthase, during oxidative stress (15). Interestingly, it has also been reported that DJ-1 enhances the activity of the transcription factor Nrf2, a master regulator of antioxidant genes (16, 17). Alternatively, DJ-1 has also been shown to modulate key signaling pathways (3, 10). One signaling pathway implicated with DJ-1 function and relevant to the present work is AKT (10, 18).
AKT is a member of a larger class of serine/threonine kinases called AGC [protein kinase A (AMP protein kinase), PKG (GMP protein kinase), and PKC]. AKT has an N-terminus pleckstrin homology domain that mediates the interaction of AKT with a plasma membrane phospholipid, phophatidylinositol 3,4,5-triphosphate (PIP3). Extensive studies have shown that recruitment of AKT to the plasma membrane, and its association with PIP3, is crucial for its activation (19, 20). Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is particularly known for its action to convert PIP3 to phosphatidylinositol-4, 5- biphosphate (PIP2). This function of PTEN directly antagonizes PI3K to eventually down-regulate AKT (21, 22). Several lines of evidence indicated that the AKT signaling pathway responds to oxidative stress (23) and exerts a neuroprotective function (24, 25). Moreover, a large number of studies in vitro have illustrated that pharmacological compounds that protect cells against oxidative stress exert their neuroprotective effects through activation of the AKT pathway (26–30).
Early studies described DJ-1 as a negative regulator of PTEN using a Drosophila genetic screen (31). Evidence to confirm this negative regulation was demonstrated via down-regulation of DJ-1 using small interfering RNA, which resulted in the inhibition of endogenous AKT phosphorylation in cancer cell lines as well as in the Drosophila brain (10, 31, 32). Furthermore, loss of DJ-1 has been shown to reduce AKT activation in response to hypoxia in murine embryonic fibroblasts (MEFs) (33). However, the relevance of this pathway has yet to be shown in the context of neurons either in vitro or in vivo. Evidence to support a role for DJ-1 in the regulation of the AKT pathway would be particularly important when one considers the genetic linkage of DJ-1 to familial PD. Presently, we provide direct evidence, both in vitro and in vivo, that DJ-1 exerts an important role in the regulation of the AKT pathway in response to oxidative stress and neuronal protection. In addition, based on our results, we propose a mechanism suggesting that DJ-1 acts as an upstream regulator of AKT through membrane recruitment to confer neuroprotection.
Results
Phosphorylation of AKT in Response to Oxidative Stress Is Reduced in the Absence of DJ-1 in Vitro and in Vivo.
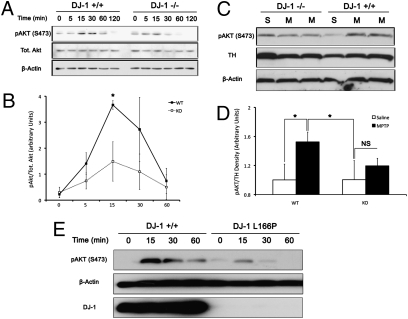
To examine the role of DJ-1 on AKT signaling, we first determined whether lack of DJ-1 affects AKT phosphorylation following hydrogen peroxide (H2O2) treatment. To test this, neurons harvested from DJ-1−/− or DJ-1+/+ embryos were treated with 100-μM H2O2 for indicated time points. As demonstrated in Fig. 1A, phosphorylation of AKT peaked in wild-type neurons at 15 min, whereas in the knockout there was a reduction in AKT phosphorylation. Quantification of three independent experiments revealed a significant reduction in p-AKT 15 min following treatment (3.67 ± 0.17 in DJ-1+/+ vs. 1.49 ± 0.76 in DJ-1−/−), as demonstrated in Fig. 1B. To further support this observation and to examine this response in a more clinically relevant model of PD, we examined AKT phosphorylation in dopaminergic neurons of the substantia nigra (SNc) in response to MPTP treatment. As indicated in Fig. 1C, and quantified in Fig. 1D, AKT phosphorylation in response to MPTP was reduced in the SNc cells of DJ-1−/− compared to wild-type controls (1.19 ± 0.10 vs. 1.52 ± 0.14, respectively). There was no significant increase in AKT phosphorylation when comparing saline and MPTP treated DJ-1−/− animals (1.00 ± 0.2 vs. 1.19 ± 0.10, respectively). To further confirm these results, we also examined AKT phosphorylation in response to H2O2 in human lymphoblasts from human PD patients harboring DJ-1 mutations. As demonstrated in Fig. 1E, AKT response was significantly attenuated in L166P mutated cells compared to the controls.
Fig. 1.
AKT activation is suppressed in the absence of DJ-1. (A) Cortical neurons from DJ-1+/+ and DJ-1−/− embryos were harvested, plated, and treated with H2O2 (100 μM) in a time-dependent fashion. Extracts were probed for pAKT (S473), total AKT, and β-actin by Western blot. (B) Quantification of A from three independent experiments. Values are presented as mean optical density relative to total AKT. (C) Eight- to 10-week-old C57Bl6 mice of WT and DJ-1 knockout genotype were treated with two 30-mg/kg doses of MPTP (M), or saline (S), given 24 h apart. Three hours following the second injection, mice brains were quickly dissected for SNc and samples were processed for Western blot analysis. (D) Quantification of C. n = 3–6 per group. (E) Immortalized lymphoblasts derived from patients with the DJ-1 L166P mutation or healthy control lymphoblasts were treated with H2O2 in a time dependant manner. Analysis of cell lysates was carried out by Western blot. Blot presented is representative of two independent experiments. Data are presented as mean ± SEM.
DJ-1 Is Necessary for AKT-Mediated Neuroprotective Function in Vitro and in Vivo.
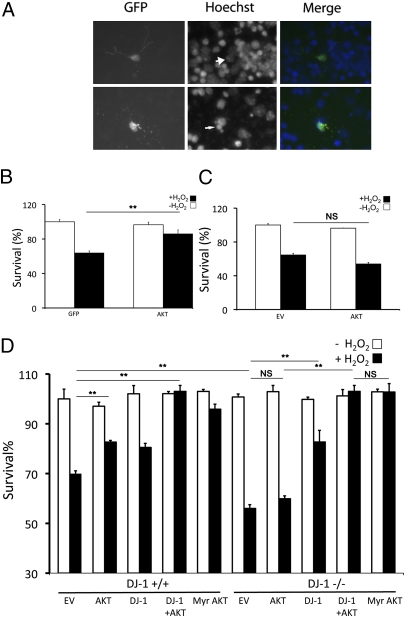
We next evaluated the functional role of DJ-1 in the protective effects of AKT following oxidative stress. First, we examined the role of AKT in protecting neurons against oxidative stress induced by H2O2 in vitro. Neurons, transfected with HA-tagged wild-type AKT along with GFP expression vectors as a marker of transfection (or GFP/empty vector transfection as control) were treated with H2O2, 24 h after transfection, and survival was assessed as described in Materials and Methods (Fig.2A). As shown in Fig. 2B, induction of exogenous wild-type AKT confers protection in DJ-1+/+ neuronal cells in response to H2O2. Next, DJ-1−/− cortical neurons were tested to examine whether induction of wild-type AKT could provide similar protection in DJ-1-deficient cells. Surprisingly, induction of exogenous AKT failed to protect DJ-1−/− neurons against H2O2-induced death (Fig. 2C). To confirm these observations, we cultured neurons harvested from DJ-1−/− and DJ-1+/+ litters at the same time. Three days after plating, the cells were transiently transfected with wild-type AKT together with or without a DJ-1 expression vector, DJ-1 only, or myristoylated AKT, a membrane-anchored constitutively active form of AKT (34). After treatment with H2O2, cell survival was assessed. The results of this experiment clearly verified our findings in Fig. 2C, indicating the protective role of wildtype AKT expressed in DJ-1+/+ neurons but not in DJ-1−/− cells (82.65 ± 0.65% DJ-1+/+ vs. 59.88 ± 1.18% DJ-1−/−) (Fig. 2D). Interestingly, myristoylated AKT significantly protects neurons against oxidative damage induced by H2O2 regardless of DJ-1 genotype (95.85 ± 2.02% DJ-1+/+ vs. 102.77 ± 3.38% DJ-1−/−).
Fig. 2.
AKT requires DJ-1 to exert its neuroprotective function in vitro. (A) Representative pictures of alive (Upper, large arrowhead) and dead (Lower, thin arrowhead) neurons. Neuronal survival was measured by identifying GFP-positive cells and determining their nuclear integrity by Hoechst stain. (B–D) Cortical neurons from either DJ-1+/+ or DJ-1−/− embryos were harvested, plated, and transfected with empty vector (EV), AKT, DJ-1, or Myr AKT. Cells were treated with H2O2 (30 μM) or vehicle control (−H2O2) for 3 h. Quantification was assessed as in A. Data are presented as mean ± SEM. **, P < 0.01; NS, no significant difference.
Suppression of AKT Abolishes the Neuroprotective Function of DJ-1 in Vitro and in Vivo.
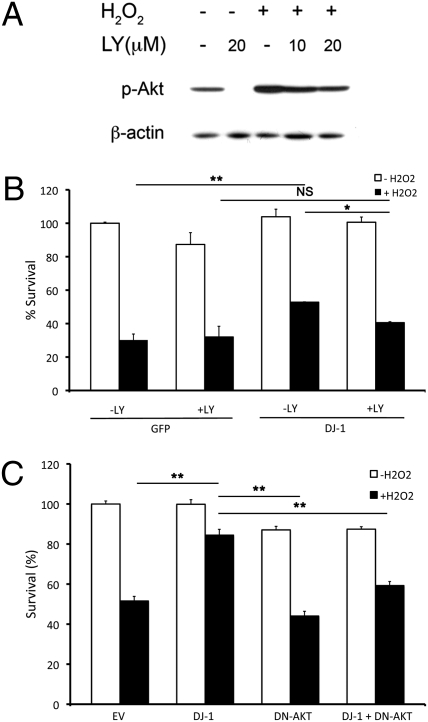
The observations that DJ-1 deficiency reduces AKT activation and that wild-type AKT requires DJ-1 to effectively protect neurons against oxidative stress suggests DJ-1 acts as an upstream activator of AKT. We next determined whether DJ-1 exerts its neuroprotective effects, at least partially, through the AKT pathway. To examine this, we first used a conventional pharmacological inhibitor of AKT, LY294002 (LY) (35). Because the basal activity of AKT is essential for the long-term health of cultured neurons, we determined the optimal dose of inhibitor that suppressed AKT with minimal toxicity to the cultured neurons (Fig. 3A). We next infected cultured cortical neurons with adenoviral vectors expressing GFP only or DJ-1 and GFP on separate promoters at the time of plating. Thirty-six hours after plating, we pretreated the cells with 10-μM LY or vehicle for 30 min before application of H2O2 or vehicle for 3 h. Cells were then assessed for survival. As shown in Fig. 3B, the neuroprotective activity of DJ-1 is significantly reduced upon suppression of AKT phosphorylation by LY (52.78 ± 0.20% vs. 40.55 ± 0.55%, respectively). We also used a more specific molecular strategy to validate our results by transiently transfecting a phosphorylation mutant, dominant-negative form (DN-AKT) of AKT (AAA-AKT) into cortical neurons. In this mutant, all phosphorylation sites of AKT have been mutated to alanine; therefore, this artificial mutant of AKT is incapable of being phosphorylated and displays dominant-negative properties toward endogenous AKT (21). As shown in Fig. 3C, the results of this experiment confirmed that suppression of AKT diminished the neuroprotective function of DJ-1 (84.46 ± 2.90% without DN-AKT vs. 59.26 ± 2.01% with DN-AKT).
Fig. 3.
DJ-1 requires AKT activation to promote cellular survival in vitro. (A) Cortical neurons were treated for either 10 or 20 μM of LY with and without H2O2 (100 μM, 15 min) to determine the effective dose of LY. (B) Cortical neurons were infected with either GFP or DJ-1 with GFP. Cells were then pretreated with LY followed by H2O2 treatment for survival assessment. (C) Cortical neurons were cotransfected with GFP and empty vector (EV), DJ-1, DN-AKT, or a DJ-1/DN-AKT combination followed by H2O2 treatment. Survival was assessed as in B. Data are presented as mean ± SEM. *, P < 0.05; **, P < 0.01; NS, no significant difference.
DJ-1 Is Necessary for AKT-Mediated Neuroprotection in Vivo Following MPTP Treatment.
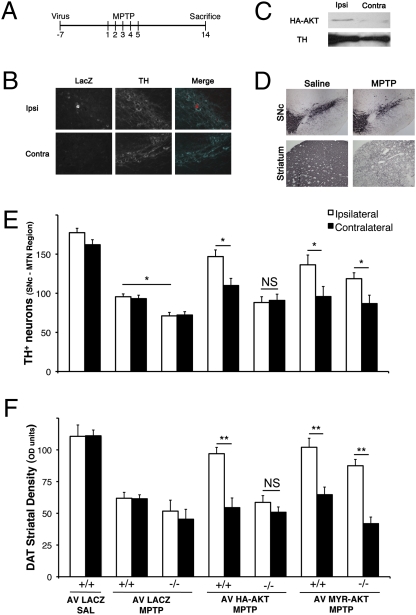
In vitro experiments indicated that DJ-1 is necessary for AKT activation and is neuroprotective in response to H2O2. To confirm these results and to test this hypothesis in a more clinically relevant paradigm, we examined whether induction of wild-type AKT can protect nigrostriatal neurons against the dopaminergic specific neurotoxin MPTP in vivo. To achieve this, we injected adenoviral vectors harboring HA-tagged wild-type AKT or myristoylated AKT into the striatum of DJ-1+/+ and DJ-1−/− age-matched mice. β-gal expressing adenoviruses were used as control. As shown in Fig. 4 B and C, the virus localizes specifically to the ipsilateral side in dopamine neurons. One week after virus injection, we performed a subchronic MPTP treatment paradigm as indicated in Fig. 4A. Two weeks after the initial MPTP injection, animals in all groups were sacrificed and prepared for histological analysis. We first assessed survival by counting the number TH+ neurons of SNc at the level of the medial terminal nucleus (MTN) (Fig. 4E). Consistent with our in vitro observations, DJ-1+/+ animals that received wild-type HA-tagged AKT and were subjected to MPTP treatments showed larger number of surviving TH+ neurons in the ipsilateral side of virus injection, compared to the contralateral side (146.9 ± 8.5 vs. 109.9 ± 9.2, respectively). Meanwhile, there was no significant difference between ipsi- and contralateral sides of the SNc in the knockout animals that received HA-AKT. (88.2 ± 7.4 vs. 90.9 ± 8.0, respectively). To verify whether the loss of TH immunoreactivity was in fact a result of the death of dopaminergic neurons and not loss of expression, we stained for cresyl violet and assessed neuronal survival in the MTN region of the SNc. Similarly, a substantial rescue was observed in the wild-type mice (62.8 ± 1.9% vs. 50.7 ± 3.0%, ipsilateral vs. contralateral), whereas no protective effect was observed when injecting HA-AKT in the DJ-1−/− mice (48.9 ± 3.0% vs. 51.9 ± 4.0% ipsilateral vs. contralateral) (Fig. S1). To further substantiate the SNc neuronal survival results, we examined whether prophylactic administration of virus could rescue dopaminergic terminals in the striatum of the animals subjected to MPTP injection in each group using expression of dopamine transporter (DAT) as a marker of dopaminergic terminals. Consistent with SNc results, higher densities, and thus greater survival of dopaminergic terminals, were observed following MPTP treatment in the striatum of virus-injected sides compared to the contralateral sides in the AKT-expressing group (97.0 ± 5.0 vs. 54.5 ± 7.6 for HA-AKT, respectively) (Fig. 4F). Such protection was not observed in DJ-1−/− animals, which signifies that the AKT-mediated neuroprotection is dependent upon the presence of DJ-1. In line with our observations in vitro and in vivo, myristoylated AKT (Myr-AKT) provides protection to both DJ-1−/− and DJ-1+/+ animals (87.5 ± 5.0 ipsilateral vs. 41.9 ± 5.2 contralateral, and 102.0 ± 7.1 ipsilateral vs. 64.7 ± 6.0 contralateral, respectively). All viruses were also injected without MPTP treatment to note effects of virus toxicity. No significant death of SNc neurons was attributed to viral vectors.
Fig. 4.
AKT requires DJ-1 to exert its neuroprotective function in an in vivo model of PD. (A) Schematic representation of treatment course. Mice were injected ipsilaterally in the striatum with adenovirus (LacZ, HA-AKT, Myr-AKT) 7 d before commencement of MPTP injections. MPTP was injected for 5 consecutive days and brains were collected 14 days following the first MPTP injection. (B) Confirmation of virus expression was performed by immunohistochemistry. Dual labeling of both TH and protein of interest in the SNc. (C) HA expression was tested in the SNc by Western blot analysis. (D) Representative pictures of both striatum and SNc of mice treated with MPTP or saline. SNc and striatum were stained for TH and DAT, respectively. (E) Quantification of TH-immunoreactive neurons was performed at the MTN region of the SNc where virus expression was highest. “Ipsi” denotes the side of the brain ipsilateral to the virus injection, whereas “contra” denotes the contralateral side. (F) Quantification of DAT-positive fibers normalized to cortex (DAT-negative). Data are presented as mean ± SEM. *, P < 0.05; **, P < 0.01; NS, no significant difference.
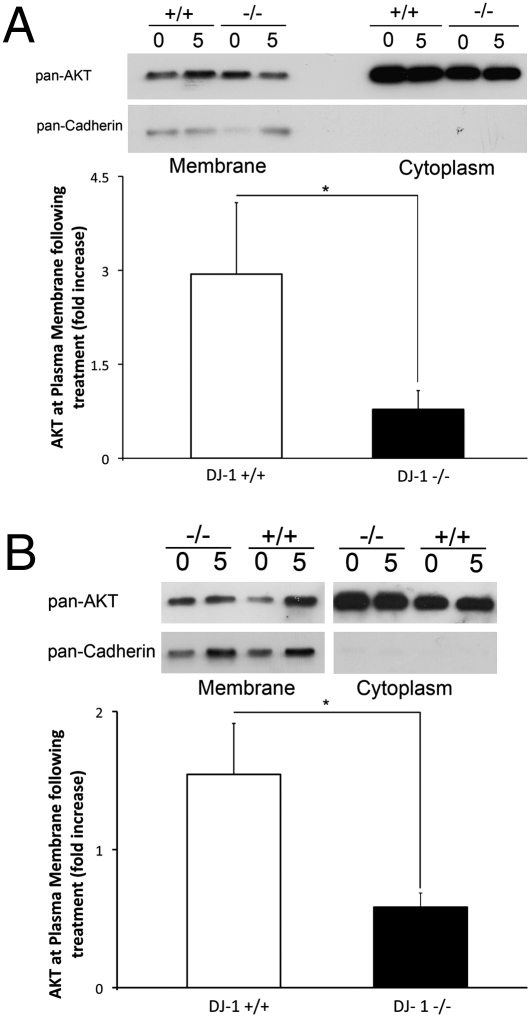
DJ-1 Modulates AKT Translocation to Membranous Fractions.
Our results in vitro and in vivo demonstrated specifically that myristoylated rather than a wild-type form of AKT promotes protection in DJ-1−/− neurons. We therefore tested whether DJ-1 was affecting AKT translocation to membranous compartments following oxidative stress. This was done by determining the subcellular localization of AKT following H2O2 treatment in DJ-1+/+ and DJ-1−/− neurons and MEFs. As shown in Fig. 5A, the H2O2-induced AKT localization to the membrane fraction is greatly decreased in the DJ-1−/− compared with the DJ-1+/+ MEFs. Quantification revealed AKT translocation to the membrane fraction following treatment that was 4-fold greater in the DJ-1+/+ than in DJ-1−/− cells (2.94 ± 1.14 vs. 0.78 ± 0.30, respectively). Similarly, in DJ-1−/− neurons, no AKT translocation was observed following H2O2 (0.58 ± 0.10-fold increase), whereas DJ-1+/+ neurons showed an AKT translocation 5 min post-treatment (1.55 ± 0.37 fold increase) No significant differences were observed in levels of total AKT in the cytoplasmic fraction.
Fig. 5.
AKT requires DJ-1 to localize to membranous fractions following oxidative insult. (A) DJ-1+/+ and DJ-1−/− MEFs were treated with 500 μM of H2O2 for 5 min or with media control. Western blot analysis by probing pan-AKT and pan-Cadherin as membranous fraction control. Quantification of membranous fractions was performed in the lower panel by calculating relative AKT density normalized to Cadherin levels and normalizing treatment group to control. Data are representative of n = 4 experiments. (B) DJ-1+/+ and DJ-1−/− cortical neurons were subjected to 100 μM of H2O2 for 5 min or media control. Quantification was performed as in A. Data are presented as mean ± SEM. *, P < 0.05.
Discussion
DJ-1 was first discovered as a weak oncogene with an unclear mechanism of action (36). Since then, putative roles for DJ-1 have been proposed, which include functions in transcriptional regulation either via binding to and modulating an androgen receptor inhibitor, PIASx-alpha (37), as well as RNA–protein interactions (38). The DJ-1 protein also displayed some homology to the proteins of the ThiJ/PfpI family of bacterial proteases (39, 40), suggesting a putative chaperone function. Interestingly, DJ-1 was also noted to display an isoelectric pH shift upon induction of oxidative stress, potentially placing DJ-1 within the oxidative stress-response pathway. Despite these important implications, its physiological relevance was not entirely clear until its genetic linkage to PD. To this end, several themes regarding DJ-1 have now emerged that link this protein to neurodegeneration, PD, and oxidative stress. These themes include, but are not limited to the following: (i) DJ-1 protects neurons against oxidative stress (4, 9, 11, 41–44); (ii) Loss of DJ-1 on its own does not lead to dopamine neuron death, at least in mice, but DJ-1-deficient animals are sensitized to environmental stress and exhibit impaired dopamine signaling (45–48); and (iii) DJ-1 modulates signaling pathways critical to cell survival such as PTEN and AKT, at least in select nonneuronal contexts (31).
In the present study, we more carefully characterized the necessity of DJ-1 for activation of the AKT pathway in response to oxidative injury, particularly in neurons. We first demonstrated that the absence of DJ-1 significantly attenuates AKT phosphorylation in vitro and in vivo, as well as in human lymphoblasts derived from PD patients harboring pathogenic DJ-1 mutations. Importantly, even though AKT phosphorylation is not completely abolished by loss of DJ-1, we also demonstrated that the significant attenuation in AKT signaling brought about by DJ-1 deficiency resulted in enhanced cell death both in vitro and in vivo. These data not only highlighted an important functional role for DJ-1 in AKT-mediated cell survival, but also indicated that the AKT pathway is integral to the mechanism of protection conferred by DJ-1 and suggested that DJ-1 could be an upstream regulator of AKT.
In light of our findings above, together with the knowledge that AKT is considered to be part of the survival pathway, we sought to further investigate the nature of the DJ-1/AKT relationship. We first demonstrated that overexpression of AKT alone protects cultured neurons exposed to oxidative stress in vitro as well as dopamine neurons exposed to MPTP in vivo. Furthermore, inhibition of the PI3K/Akt pathway significantly reduces the protection that is conferred by DJ-1. Importantly, we also demonstrated that wild-type AKT required DJ-1 to exert its protective effect as DJ-1 deficiency abrogated the effect of AKT on cell survival. Interestingly, the protective effects of AKT in a DJ-1-deficient background can be bypassed using myristoylated AKT, its membrane-anchored constitutively active form. This latter observation is consistent with reports that membrane-bound AKT is sufficient to provide protection following MPP+ treatment both in vitro (49) as well as 6-OHDA treatment in vivo (50). Because AKT recruitment to the membrane is a prior event to its phosphorylation and activation (51, 52), these results, in addition to the cell fractionation experiments presented in our study, suggested that DJ-1 permits AKT translocation to the membrane fractions.
Our study therefore proposes a working model in which DJ-1 acts upstream of AKT, thereby facilitating its activation following neuronal injury via oxidative stress. We propose that DJ-1 may be involved in fine-tuning the response of neurons to ROS and modulation of signaling pathways that mediate survival. In this regard, it will be critical in future studies to address the possible mechanisms underlying the ROS-mediated, DJ-1-dependent activation of AKT. One might consider the possibility that DJ-1 regulates AKT by modulating its recruitment to the membrane in a ROS-dependent manner. It is noteworthy that the AKT response to H2O2 can be altered depending on antioxidant protein activity within the cell (53–55). Thus, further studies in models that permit well-controlled ROS levels are needed to address these questions. However, other possibilities exist. For example, a recent study has suggested that DJ-1 interacts with PTEN to permit AKT activation, although this needs to be further investigated in more physiologically relevant models (56). Additionally, DJ-1 may interact with other PI3K pathway kinases, such as mTOR and PDK, to permit AKT phosphorylation. Finally, while DJ-1 plays a significant role in facilitating AKT phosphorylation, other factors may also each play a role (21, 57, 58). Thus, additional studies should be performed to investigate the nature of the DJ-1/AKT interdependence.
Finally, it is interesting to note that even though it is clear that DJ-1 is linked to familial PD, there is a report of an epidemiological association with certain haplotype of AKT1 and a reduced risk of PD (59). This observation provides further strength to the notion that the DJ-1/AKT signaling axis may be important in regulating dopaminergic function or death. Elucidation of these mechanisms may provide an eventual basis for neuroprotective therapies.
Materials and Methods
Cell culture, Western blot analysis, and in vivo stereotaxic injections and MPTP administration were performed as previously described (60). All procedures involving animals were approved by the University of Ottawa Animal Care Committee and were maintained in strict accordance with the Guidelines for the Use and Treatment of Animals put forth by the Animal Care Council of Canada and endorsed by the Canadian Institutes of Health Research. For additional in vivo and in vitro procedures, see SI Materials and Methods.
Subcellular Fractionation.
Membrane fractions were obtained similarly for MEFs and DIV 6 cortical neurons using differential centrifugation. Briefly, cells were harvested in cold PBS and centrifuged at 1,200 × g for 3 min. The cell pellet was resuspended in 200 μL of hypo-osmolar buffer [50 mM Tris-HCl, pH 7.4; 50 mM NaCl; protease inhibitor complex (Roche)] and homogenized for 30 s. Samples were centrifuged at 20,000 × g, at 4 °C for 20 min. Supernatants (cellular debris) were transferred to 1.5-mL ultracentrifuge tubes (Beckman) and centrifuged at 100,000 × g, at 4 °C for 3 h. The pellets (microsomal enriched) were resuspended in RIPA buffer (150 mM NaCl; 1% Nonidet P-40; 0.5% deoxycholic acid; 0.1% SDS; 50 mM Tris-HCl, pH 8.0) and sonicated briefly for subsequent Western blot analysis. Supernatants from final spin were used as a cytoplasmic control.
Statistical Analysis.
Statistical significance was either determined by Student’s t-test or one-way ANOVA followed by Tukey’s post hoc test. All data are presented as mean ± SEM. Significance at P < 0.05 (*) and P < 0.01 (**), and NS denotes no significant difference.
Supplementary Material
Acknowledgments
This work was supported by grants from Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, the Canadian Stroke Network, The Centre for Stroke Recovery, Parkinson Society Canada, and Parkinson’s Disease Foundation (to D.S.P), Heart and Stroke Foundation of Canada (to H.A.), Heart and Stroke Foundation of Ontario (to M.W.C.R.), Canadian Institutes of Health Research (to I.I.), and the Parkinson Society Canada (to S.J.H.), and the World Class University program through the National Research Foundation of Korea (Grant R31-2008-000-20004-0). Adenoviral constructs were provided by Dr. J. Albrecht at the Hennepin County Medical Center (Minneapolis, MN).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914876107/DCSupplemental.
References
- 1.Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol. 2003;54:283–286. doi: 10.1002/ana.10675. [DOI] [PubMed] [Google Scholar]
- 3.Gu L, et al. Involvement of ERK1/2 signaling pathway in DJ-1-induced neuroprotection against oxidative stress. Biochem Biophys Res Commun. 2009;383:469–474. doi: 10.1016/j.bbrc.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Inden M, et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol Dis. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Lev N, et al. DJ-1 protects against dopamine toxicity. J Neural Transm. 2009;116:151–160. doi: 10.1007/s00702-008-0134-4. [DOI] [PubMed] [Google Scholar]
- 6.Canet-Avilés RM, et al. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taira T, et al. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim RH, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavara-Culebras E, Paricio N. Drosophila DJ-1 mutants are sensitive to oxidative stress and show reduced lifespan and motor deficits. Gene. 2007;400:158–165. doi: 10.1016/j.gene.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinat C, et al. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aleyasin H, et al. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci USA. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andres-Mateos E, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, et al. The Parkinson’s disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum Mol Genet. 2005;14:1231–1241. doi: 10.1093/hmg/ddi134. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra D, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Brug MP, et al. RNA binding activity of the recessive Parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci USA. 2008;105:10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klippel A, Kavanaugh WM, Pot D, Williams LT. A specific product of phosphatidylino-sitol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohn AD, Takeuchi F, Roth RA. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 21.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crossthwaite AJ, Hasan S, Williams RJ. Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and JNK in cortical neurones: dependence on Ca(2+) and PI3-kinase. J Neurochem. 2002;80:24–35. doi: 10.1046/j.0022-3042.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, et al. Insulin/PI3K signaling protects dentate neurons from oxygen-glucose deprivation in organotypic slice cultures. J Neurochem. 2009;112:377–388. doi: 10.1111/j.1471-4159.2009.06450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HJ, Kim MK, Kim HJ, Kim SU. Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model. PLoS One. 2009;4:e5586. doi: 10.1371/journal.pone.0005586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Hu Y, Zhu Q, Zhu J. Neurotrophin-3 reduces apoptosis induced by 6-OHDA in PC12 cells through Akt signaling pathway. Int J Dev Neurosci. 2008;26:635–640. doi: 10.1016/j.ijdevneu.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Dudek H, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 28.Malagelada C, Jin ZH, Greene LA. RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J Neurosci. 2008;28:14363–14371. doi: 10.1523/JNEUROSCI.3928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heo SR, Han AM, Kwon YK, Joung I. p62 protects SH-SY5Y neuroblastoma cells against H2O2-induced injury through the PDK1/Akt pathway. Neurosci Lett. 2009;450:45–50. doi: 10.1016/j.neulet.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Liu JH, Yin F, Guo LX, Deng XH, Hu YH. Neuroprotection of geniposide against hydrogen peroxide induced PC12 cells injury: involvement of PI3 kinase signal pathway. Acta Pharmacol Sin. 2009;30:159–165. doi: 10.1038/aps.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim RH, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Sitaram RT, et al. The PTEN regulator DJ-1 is associated with hTERT expression in clear cell renal cell carcinoma. Int J Cancer. 2009;125:783–790. doi: 10.1002/ijc.24335. [DOI] [PubMed] [Google Scholar]
- 33.Vasseur S, et al. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci USA. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier R, Alessi DR, Cron P, Andjelković M, Hemmings BA. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 35.Taylor JM, et al. Akt phosphorylation and NFkappaB activation are counterregulated under conditions of oxidative stress. Exp Cell Res. 2004;300:463–475. doi: 10.1016/j.yexcr.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Nagakubo D, et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, et al. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J Biol Chem. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- 38.Hod Y, Pentyala SN, Whyard TC, El-Maghrabi MR. Identification and characterization of a novel protein that regulates RNA-protein interaction. J Cell Biochem. 1999;72:435–444. [PubMed] [Google Scholar]
- 39.Mitsumoto A, et al. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 40.Wilson MA, St Amour CV, Collins JL, Ringe D, Petsko GA. The 1.8-A resolution crystal structure of YDR533Cp from Saccharomyces cerevisiae: a member of the DJ-1/ThiJ/PfpI superfamily. Proc Natl Acad Sci USA. 2004;101:1531–1536. doi: 10.1073/pnas.0308089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, et al. Parkin regulates Eg5 expression by Hsp70 ubiquitination-dependent inactivation of c-Jun NH2-terminal kinase. J Biol Chem. 2008;283:35783–35788. doi: 10.1074/jbc.M806860200. [DOI] [PubMed] [Google Scholar]
- 42.Paterna JC, Leng A, Weber E, Feldon J, Büeler H. DJ-1 and Parkin modulate dopamine-dependent behavior and inhibit MPTP-induced nigral dopamine neuron loss in mice. Mol Ther. 2007;15:698–704. doi: 10.1038/sj.mt.6300067. [DOI] [PubMed] [Google Scholar]
- 43.Meulener M, et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 44.Yokota T, et al. Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem Biophys Res Commun. 2003;312:1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi H, Shen J. Absence of dopaminergic neuronal degeneration and oxidative damage in aged DJ-1-deficient mice. Mol Neurodegener. 2007;2:10. doi: 10.1186/1750-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pisani A, et al. Enhanced sensitivity of DJ-1-deficient dopaminergic neurons to energy metabolism impairment: role of Na+/K+ ATPase. Neurobiol Dis. 2006;23:54–60. doi: 10.1016/j.nbd.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Park J, et al. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg MS, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 49.Salinas M, Martín D, Alvarez A, Cuadrado A. Akt1/PKBalpha protects PC12 cells against the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium and reduces the levels of oxygen-free radicals. Mol Cell Neurosci. 2001;17:67–77. doi: 10.1006/mcne.2000.0921. [DOI] [PubMed] [Google Scholar]
- 50.Ries V, et al. Oncoprotein Akt/PKB induces trophic effects in murine models of Parkinson’s disease. Proc Natl Acad Sci USA. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James SR, et al. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315:709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 53.Taylor JM, Ali U, Iannello RC, Hertzog P, Crack PJ. Diminished Akt phosphorylation in neurons lacking glutathione peroxidase-1 (Gpx1) leads to increased susceptibility to oxidative stress-induced cell death. J Neurochem. 2005;92:283–293. doi: 10.1111/j.1471-4159.2004.02863.x. [DOI] [PubMed] [Google Scholar]
- 54.Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3beta survival signaling. J Cereb Blood Flow Metab. 2007;27:975–982. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Handy DE, et al. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim YC, Kitaura H, Taira T, Iguchi-Ariga SM, Ariga H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int J Oncol. 2009;35:1331–1341. [PubMed] [Google Scholar]
- 57.Alessi DR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 58.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 59.Xiromerisiou G, et al. Association between AKT1 gene and Parkinson’s disease: a protective haplotype. Neurosci Lett. 2008;436:232–234. doi: 10.1016/j.neulet.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu D, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.